Abstract
Rationale
Therapeutic approaches to mitigating traumatic memories have often faced resistance. Exploring safe reconsolidation blockers, drugs capable of reducing the emotional valence of the memory upon brief retrieval and reactivation, emerges as a promising pharmacological strategy. Towards this objective, preclinical investigations should focus on aversive memories resulting in maladaptive outcomes and consider sex-related differences to enhance their translatability.
Objectives
After selecting a relatively high training magnitude leading to the formation of a more intense and generalized fear memory in adult female and male rats, we investigated whether two clinically approved drugs disrupting its reconsolidation remain effective.
Results
We found resistant reconsolidation impairment by the α2-adrenergic receptor agonist clonidine or cannabidiol, a major non-psychotomimetic Cannabis sativa component. However, pre-retrieval administration of D-cycloserine, a partial agonist at the glycine-binding site of the N-methyl-D-aspartate (NMDA) receptor complex, facilitated their impairing effects on reconsolidation. A similar reconsolidation blockade by clonidine or cannabidiol was achieved following exposure to a non-conditioned but generalized context after D-cycloserine administration. This suggests that sufficient memory destabilization can accompany generalized fear expression. Combining clonidine with cannabidiol without potentiating memory destabilization by D-cycloserine was ineffective.
Conclusions
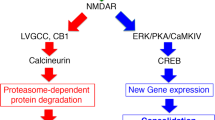
These findings highlight the importance of NMDA receptor signaling in memory destabilization and underscore the efficacy of a dual-step pharmacological intervention in attenuating traumatic-like memories, even in a context different from the original learning environment.
Graphical Abstract






Similar content being viewed by others
Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.
References
Asim M, Hao B, Yang YH, Fan BF, Xue L, Shi YW, Wang XG, Zhao H (2020) Ketamine Alleviates Fear Generalization Through GluN2B-BDNF Signaling in Mice. Neurosci Bull 36(2):153–164. https://doi.org/10.1007/s12264-019-00422-4
Baldi E, Lorenzini CA, Bucherelli C (2004) Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem 81(3):162–166. https://doi.org/10.1016/j.nlm.2004.02.004
Barker JM, Galea LA (2010) Males show stronger contextual fear conditioning than females after context pre-exposure. Physiol Behav 99(1):82–90. https://doi.org/10.1016/j.physbeh.2009.10.014
Bayer H, Stern CAJ, Troyner F, Gazarini L, Guimarães FS, Bertoglio LJ (2022) Medial prefrontal cortex mechanisms of cannabidiol-induced aversive memory reconsolidation impairments. Neuropharmacology 205:108913. https://doi.org/10.1016/j.neuropharm.2021.108913
Ben Mamou C, Gamache K, Nader K (2006) NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci 9(10):1237–1239. https://doi.org/10.1038/nn1778
Blanchard RJ, Blanchard DC (1969) Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol 68(1):129–35. https://doi.org/10.1037/h0027676
Bryant RA, Felmingham KL, Silove D, Creamer M, O’Donnell M, McFarlane AC (2011) The association between menstrual cycle and traumatic memories. J Affect Disord 131(1–3):398–401. https://doi.org/10.1016/j.jad.2010.10.049
Bustos SG, Giachero M, Maldonado H, Molina VA (2010) Previous stress attenuates the susceptibility to Midazolam’s disruptive effect on fear memory reconsolidation: influence of pre-reactivation D-cycloserine administration. Neuropsychopharmacology 35(5):1097–1108. https://doi.org/10.1038/npp.2009.215
Cain CK, Blouin AM, Barad M (2004) Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem 11(2):179–187. https://doi.org/10.1101/lm.71504
Couto-Pereira NS, Lampert C, Vieira ADS, Lazzaretti C, Kincheski GC, Espejo PJ, Molina VA, Quillfeldt JA, Dalmaz C (2019) Resilience and Vulnerability to Trauma: Early Life Interventions Modulate Aversive Memory Reconsolidation in the Dorsal Hippocampus. Front Mol Neurosci 12:134. https://doi.org/10.3389/fnmol.2019.00134
Crestani AP, Zacouteguy Boos F, Haubrich J, Ordoñez Sierra R, Santana F, Molina JM, Cassini Lde F, Alvares Lde O, Quillfeldt JÁ (2015) Memory reconsolidation may be disrupted by a distractor stimulus presented during reactivation. Sci Rep 5:13633. https://doi.org/10.1038/srep13633
da Silva TR, Takahashi RN, Bertoglio LJ, Andreatini R, Stern CA (2016) Evidence for an expanded time-window to mitigate a reactivated fear memory by tamoxifen. Eur Neuropsychopharmacol 26(10):1601–1609. https://doi.org/10.1016/j.euroneuro.2016.08.005
Day HLL, Stevenson CW (2020) The neurobiological basis of sex differences in learned fear and its inhibition. Eur J Neurosci 52(1):2466–2486. https://doi.org/10.1111/ejn.14602
Dos Santos CM, Vaz BDS, Grisanti GDV, de Paiva JPQ, Tiba PA, Fornari RV (2019) Relationship between footshock intensity, post-training corticosterone release and contextual fear memory specificity over time. Psychoneuroendocrinology 110:104447. https://doi.org/10.1016/j.psyneuen.2019.104447
Espejo PJ, Ortiz V, Martijena ID, Molina VA (2016) Stress-induced resistance to the fear memory labilization/reconsolidation process. Involvement of the basolateral amygdala complex. Neuropharmacology 109:349–356. https://doi.org/10.1016/j.neuropharm.2016.06.033
Ferrer Monti RI, Giachero M, Alfei JM, Bueno AM, Cuadra G, Molina VA (2016) An appetitive experience after fear memory destabilization attenuates fear retention: involvement GluN2B-NMDA receptors in the Basolateral Amygdala Complex. Learn Mem 23(9):465–478. https://doi.org/10.1101/lm.042564.116
Flavell CR, Gascoyne RM, Lee JLC (2020) Postreactivation mifepristone impairs generalization of strongly conditioned contextual fear memories. Learn Mem 27(12):483–487. https://doi.org/10.1101/lm.052167.120
Franzen JM, Giachero M, Bertoglio LJ (2019) Dissociating retrieval-dependent contextual aversive memory processes in female rats: Are there cycle-dependent differences? Neuroscience 406:542–553. https://doi.org/10.1016/j.neuroscience.2019.03.035
Franzen JM, Vanz F, Werle I, Guimarães FS, Bertoglio LJ (2022) Cannabidiol impairs fear memory reconsolidation in female rats through dorsal hippocampus CB1 but not CB2 receptor interaction. Eur Neuropsychopharmacol 64:7–18. https://doi.org/10.1016/j.euroneuro.2022.08.002
Franzen JM, Werle I, Vanz F, de Oliveira BB, Martins Nascimento LM, Guimarães FS, Bertoglio LJ (2023) Cannabidiol attenuates fear memory expression in female rats via hippocampal 5-HT1A but not CB1 or CB2 receptors. Neuropharmacology 223:109316. https://doi.org/10.1016/j.neuropharm.2022.109316
Gamache K, Pitman RK, Nader K (2012) Preclinical evaluation of reconsolidation blockade by clonidine as a potential novel treatment for posttraumatic stress disorder. Neuropsychopharmacology 37(13):2789–2796. https://doi.org/10.1038/npp.2012.145
Gazarini L, Stern CA, Carobrez AP, Bertoglio LJ (2013) Enhanced noradrenergic activity potentiates fear memory consolidation and reconsolidation by differentially recruiting α1- and β-adrenergic receptors. Learn Mem 20(4):210–219. https://doi.org/10.1101/lm.030007.112
Gazarini L, Stern CA, Piornedo RR, Takahashi RN, Bertoglio LJ (2014) PTSD-like memory generated through enhanced noradrenergic activity is mitigated by a dual step pharmacological intervention targeting its reconsolidation. Int J Neuropsychopharmacol 18(1):pyu026. https://doi.org/10.1093/ijnp/pyu026
Gazarini L, Stern CA, Takahashi RN, Bertoglio LJ (2022) Interactions of Noradrenergic, Glucocorticoid and Endocannabinoid Systems Intensify and Generalize Fear Memory Traces. Neuroscience 497:118–133. https://doi.org/10.1016/j.neuroscience.2021.09.012
Gazarini L, Stern CAJ, Bertoglio LJ (2023) On making (and turning adaptive to) maladaptive aversive memories in laboratory rodents. Neurosci Biobehav Rev 147:105101. https://doi.org/10.1016/j.neubiorev.2023.105101
Gresack JE, Schafe GE, Orr PT, Frick KM (2009) Sex differences in contextual fear conditioning are associated with differential ventral hippocampal extracellular signal-regulated kinase activation. Neuroscience 159(2):451–467. https://doi.org/10.1016/j.neuroscience.2009.01.009
Hall J, Thomas KL, Everitt BJ (2001) Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci 21(6):2186–2193. https://doi.org/10.1523/JNEUROSCI.21-06-02186.2001
Han X, Song X, Song D, Xie G, Guo H, Wu N, Li J (2022) Comparison between cannabidiol and sertraline for the modulation of post-traumatic stress disorder-like behaviors and fear memory in mice. Psychopharmacology 239(5):1605–1620. https://doi.org/10.1007/s00213-022-06132-6
Haubrich J, Bernabo M, Nader K (2020) Noradrenergic projections from the locus coeruleus to the amygdala constrain fear memory reconsolidation. Elife 9:e57010. https://doi.org/10.7554/eLife.57010
Haubrich J, Nader K (2023) Network-level changes in the brain underlie fear memory strength. Elife 12:RP88172. https://doi.org/10.7554/eLife.88172
Holehonnur R, Phensy AJ, Kim LJ, Milivojevic M, Vuong D, Daison DK, Alex S, Tiner M, Jones LE, Kroener S, Ploski JE (2016) Increasing the GluN2A/GluN2B Ratio in Neurons of the Mouse Basal and Lateral Amygdala Inhibits the Modification of an Existing Fear Memory Trace. J Neurosci 36(36):9490–9504. https://doi.org/10.1523/JNEUROSCI.1743-16.2016
Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, Tronson NC (2017) Sex Differences in Context Fear Generalization and Recruitment of Hippocampus and Amygdala during Retrieval. Neuropsychopharmacology 42(2):397–407. https://doi.org/10.1038/npp.2016.174
Kindt M, van Emmerik A (2016) New avenues for treating emotional memory disorders: towards a reconsolidation intervention for posttraumatic stress disorder. Ther Adv Psychopharmacol 6(4):283–295. https://doi.org/10.1177/2045125316644541
Ledgerwood L, Richardson R, Cranney J (2003) Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci 117(2):341–349. https://doi.org/10.1037/0735-7044.117.2.341
Lee JL, Milton AL, Everitt BJ (2006) Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci 26(39):10051–10056. https://doi.org/10.1523/JNEUROSCI.2466-06.2006
Marcondes FK, Bianchi FJ, Tanno AP (2002) Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62(4A):609–614. https://doi.org/10.1590/s1519-69842002000400008
Maren S, De Oca B, Fanselow MS (1994) Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res 661(1–2):25–34. https://doi.org/10.1016/0006-8993(94)91176-2
Marin FN, Franzen JM, Troyner F, Molina VA, Giachero M, Bertoglio LJ (2020) Taking advantage of fear generalization-associated destabilization to attenuate the underlying memory via reconsolidation intervention. Neuropharmacology 181:108338. https://doi.org/10.1016/j.neuropharm.2020.108338
Milton AL, Merlo E, Ratano P, Gregory BL, Dumbreck JK, Everitt BJ (2013) Double dissociation of the requirement for GluN2B- and GluN2A-containing NMDA receptors in the destabilization and restabilization of a reconsolidating memory. J Neurosci 33(3):1109–1115. https://doi.org/10.1523/JNEUROSCI.3273-12.2013
Misanin JR, Miller RR, Lewis DJ (1968) Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 160(3827):554–555. https://doi.org/10.1126/science.160.3827.554
Mitchell JR, Trettel SG, Li AJ, Wasielewski S, Huckleberry KA, Fanikos M, Golden E, Laine MA, Shansky RM (2022) Darting across space and time: parametric modulators of sex-biased conditioned fear responses. Learn Mem 29(7):171–180. https://doi.org/10.1101/lm.053587.122
Monfils MH, Cowansage KK, Klann E, LeDoux JE (2009) Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324(5929):951–955. https://doi.org/10.1126/science.1167975
Montoya ZT, Uhernik AL, Smith JP (2020) Comparison of cannabidiol to citalopram in targeting fear memory in female mice. J Cannabis Res 2(1):48. https://doi.org/10.1186/s42238-020-00055-9
Morris RW, Bouton ME (2007) The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci 121(3):501–514. https://doi.org/10.1037/0735-7044.121.3.501
Murkar A, Kent P, Cayer C, James J, Durst T, Merali Z (2019) Cannabidiol and the Remainder of the Plant Extract Modulate the Effects of Δ9-Tetrahydrocannabinol on Fear Memory Reconsolidation. Front Behav Neurosci 13:174. https://doi.org/10.3389/fnbeh.2019.00174
Nader K, Schafe GE, Le Doux JE (2000) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406(6797):722–726. https://doi.org/10.1038/35021052
Ortiz V, Espejo PJ, Molina VA, Martijena ID (2019) Ethanol withdrawal limits fear memory reactivation-induced molecular events associated with destabilization phase: Influence of d-cycloserine. Prog Neuropsychopharmacol Biol Psychiatry 89:9–15. https://doi.org/10.1016/j.pnpbp.2018.08.018
Pedraza LK, Sierra RO, Boos FZ, Haubrich J, Quillfeldt JA, Alvares Lde O (2016) The dynamic nature of systems consolidation: Stress during learning as a switch guiding the rate of the hippocampal dependency and memory quality. Hippocampus 26(3):362–371. https://doi.org/10.1002/hipo.22527
Penninx BW, Pine DS, Holmes EA, Reif A (2021) Anxiety disorders. Lancet 397(10277):914–927. https://doi.org/10.1016/S0140-6736(21)00359-7
Pigeon S, Lonergan M, Rotondo O, Pitman RK, Brunet A (2022) Impairing memory reconsolidation with propranolol in healthy and clinical samples: a meta-analysis. J Psychiatry Neurosci 47(2):E109–E122. https://doi.org/10.1503/jpn.210057
Poulos AM, Mehta N, Lu B, Amir D, Livingston B, Santarelli A, Zhuravka I, Fanselow MS (2016) Conditioning- and time-dependent increases in context fear and generalization. Learn Mem 23(7):379–385. https://doi.org/10.1101/lm.041400.115
Przybyslawski J, Sara SJ (1997) Reconsolidation of memory after its reactivation. Behav Brain Res 84(1–2):241–246. https://doi.org/10.1016/s0166-4328(96)00153-2
Przybyslawski J, Roullet P, Sara SJ (1999) Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci 19(15):6623–6628. https://doi.org/10.1523/JNEUROSCI.19-15-06623.1999
Saggu S, Chen Y, Cottingham C, Rehman H, Wang H, Zhang S, Augelli-Szafran C, Lu S, Lambert N, Jiao K, Lu XY, Wang Q (2023) Activation of a novel α2AAR-spinophilin-cofilin axis determines the effect of α2 adrenergic drugs on fear memory reconsolidation. Mol Psychiatry 28(2):588–600. https://doi.org/10.1038/s41380-022-01851-w
Saitoh A, Akagi K, Oka JI, Yamada M (2017) Post-reexposure administration of D-cycloserine facilitates reconsolidation of contextual conditioned fear memory in rats. J Neural Transm 124(5):583–587. https://doi.org/10.1007/s00702-017-1704-0
Schade S, Paulus W (2016) D-Cycloserine in Neuropsychiatric Diseases: A Systematic Review. Int J Neuropsychopharmacol 19(4):pyv102. https://doi.org/10.1093/ijnp/pyv102
Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA (2010) Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463(7277):49–53. https://doi.org/10.1038/nature08637
Shehata M, Abdou K, Choko K, Matsuo M, Nishizono H, Inokuchi K (2018) Autophagy Enhances Memory Erasure through Synaptic Destabilization. J Neurosci 38(15):3809–3822. https://doi.org/10.1523/JNEUROSCI.3505-17.2018
Siegmund A, Wotjak CT (2007) A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J Psychiatr Res 41(10):848–860. https://doi.org/10.1016/j.jpsychires.2006.07.017
Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ (2015) Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacol Ther 149:150–190. https://doi.org/10.1016/j.pharmthera.2014.12.004
Soni M, Curran VH, Kamboj SK (2013) Identification of a narrow post-ovulatory window of vulnerability to distressing involuntary memories in healthy women. Neurobiol Learn Mem 104:32–38. https://doi.org/10.1016/j.nlm.2013.04.003
Stern CA, Gazarini L, Takahashi RN, Guimarães FS, Bertoglio LJ (2012) On disruption of fear memory by reconsolidation blockade: evidence from cannabidiol treatment. Neuropsychopharmacology 37(9):2132–2142. https://doi.org/10.1038/npp.2012.63
Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S (2004) Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24(20):4787–4795. https://doi.org/10.1523/JNEUROSCI.5491-03.2004
Troyner F, Bertoglio LJ (2020) Thalamic nucleus reuniens regulates fear memory destabilization upon retrieval. Neurobiol Learn Mem 175:107313. https://doi.org/10.1016/j.nlm.2020.107313
Troyner F, Bertoglio LJ (2021) Nucleus reuniens of the thalamus controls fear memory reconsolidation. Neurobiol Learn Mem 177:107343. https://doi.org/10.1016/j.nlm.2020.107343
Walsh KH, Das RK, Saladin ME, Kamboj SK (2018) Modulation of naturalistic maladaptive memories using behavioural and pharmacological reconsolidation-interfering strategies: a systematic review and meta-analysis of clinical and “sub-clinical” studies. Psychopharmacology 235(9):2507–2527. https://doi.org/10.1007/s00213-018-4983-8
Wang SH, de Oliveira AL, Nader K (2009) Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci 12(7):905–912. https://doi.org/10.1038/nn.2350
Wood NE, Rosasco ML, Suris AM, Spring JD, Marin MF, Lasko NB, Goetz JM, Fischer AM, Orr SP, Pitman RK (2015) Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: three negative psychophysiological studies. Psychiatry Res 225(1–2):31–39. https://doi.org/10.1016/j.psychres.2014.09.005
Wu IT, Tang TH, Ko MC, Chiu CY, Lu KT (2015) Amygdaloid zif268 participated in the D-cycloserine facilitation effect on the extinction of conditioned fear. Psychopharmacology 232(20):3809–3819. https://doi.org/10.1007/s00213-015-4042-7
Acknowledgements
This study was supported by Brazilian grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 304851/2022-1) and FAPESP (2017/24304-0), which had no other role in the design of the study, collection, and analysis of data and decision to submit the paper for publication.
Funding
This study was supported by Brazilian grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 304851/2022–1) and FAPESP (2017/24304–0), which had no other role in the design of the study, data collection and analysis, and decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
L.A.S.: conceptualization, investigation, methodology, and draft writing; L.M.M.N.: investigation; F.S.G.: funding acquisition and draft writing; L.G.: conceptualization, supervision, and draft writing; and L.J.B.: conceptualization, data curation, formal analysis, funding acquisition, project administration, resources, supervision, and writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
Francisco S. Guimarães is a co-inventor (Mechoulam R, JC, Guimaraes FS, AZ, JH, Breuer A) of the patent "Fluorinated CBD compounds, compositions and uses. Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023" Def. US no. Reg. 62193296; 29/07/2015; INPI on 19/08/2015 (BR1120150164927). The University of São Paulo has licensed the patent to Phytecs Pharm (USP Resolution No. 15.1.130002.1.1). The University of São Paulo has an agreement with Prati-Donaduzzi (Toledo, Brazil) to "develop a pharmaceutical product containing synthetic cannabidiol and prove its safety and therapeutic efficacy in the treatment of epilepsy, schizophrenia, Parkinson's disease, and anxiety disorders". The other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chemical compounds used in this article: .
• Cannabidiol (PubChem CID: 644019).
• Clonidine (PubChem CID: 2803).
• D-cycloserine (PubChem CID: 6234).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soares, L.A., Nascimento, L.M.M., Guimarães, F.S. et al. Dual-step pharmacological intervention for traumatic-like memories: implications from D-cycloserine and cannabidiol or clonidine in male and female rats. Psychopharmacology (2024). https://doi.org/10.1007/s00213-024-06596-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00213-024-06596-8