Abstract
Rationale
Preeclampsia is a condition that can affect the health in offspring at adult life. The effect on several systems has been described, but less is known about its effect on neuropsychiatric disorders at early ages.
Objective
Evaluate the possible relationship of preeclampsia with development of anxiety- and depressive-like behavior, as well as memory impairments in male and female early adolescent offspring from preeclamptic mice.
Methods
Thirty pregnant females were divided into control group receiving vehicle, and preeclampsia group receiving L-NAME in drinking water at a dose of 60 mg/Kg from day 10 of pregnancy until delivery. Offspring was weaned and sexed at 4 weeks after birth. Each group was evaluated using the elevated plus maze test (anxiety- like response), tail suspension test (depressive-like behavior) and the recognition of novel objects test (recognition memory), in addition to the open field test was performance to corroborate their motor activity and validate our results.
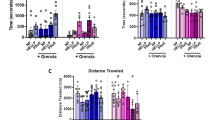
Results
We found that preeclampsia produces behavioral alterations in offspring, and this effect is dependent on sex. The male offspring from preeclampsia showed an enhancement in the time that mice spend in the close arms in the elevated plus maze test, and longer immobility time in the tail suspension test, compared to the offspring from healthy pregnancies. On the other hand, female offspring from preeclampsia showed a lower percentage of recognition in the memory test compared to offspring from normal pregnancy.
Conclusions
These results suggest that preeclampsia predisposes early adolescent young male offspring to develop anxiety- and depressive-like behavior as well as memory impairment in early adolescent young female offspring.






Similar content being viewed by others
References
Barbie-Shoshani Y, Shoham S, Bejar C, Weinstock M (2016) Sex-specific effects of prenatal stress on memory and markers of neuronal activity in juvenile rats. Dev Neurosci 38(3). https://doi.org/10.1159/000446981
Bertaina-Anglade V, Enjuanes E, Morillon D, la Drieu C (2006) The object recognition task in rats and mice: a simple and rapid model in safety pharmacology to detect amnesic properties of a new chemical entity. J Pharmacol Toxicol Methods 54(2). https://doi.org/10.1016/j.vascn.2006.04.001
Bolte AC, Van Geijn HP, Dekker GA (2001) Pathophysiology of preeclampsia and the role of serotonin. Eur J Obstet Gynecol Reproductive Biology (Vol 95(1). https://doi.org/10.1016/S0301-2115(00)00367-5
Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, Levitt P (2011) A transient placental source of serotonin for the fetal forebrain. Nature 472(7343). https://doi.org/10.1038/nature09972
Brust V, Schindler PM, Lewejohann L (2015) Lifetime development of behavioural phenotype in the house mouse (Mus musculus). In Frontiers in Zoology (Vol. 12, Issue 1). https://doi.org/10.1186/1742-9994-12-S1-S17
Cauli O, Herraiz S, Pellicer B, Pellicer A, Felipo V (2010) Treatment with sildenafil prevents impairment of learning in rats born to pre-eclamptic mothers. Neuroscience 171(2). https://doi.org/10.1016/j.neuroscience.2010.08.065
Cheng SW, Chou HC, Tsou KI, Fang LJ, Tsao PN (2004) Delivery before 32 weeks of gestation for maternal pre-eclampsia: neonatal outcome and 2-year developmental outcome. Early Hum Dev 76(1). https://doi.org/10.1016/j.earlhumdev.2003.10.004
Dachew BA, Mamun A, Maravilla JC, Alati R (2018) Pre-eclampsia and the risk of autism-spectrum disorder in offspring: Meta-analysis. Br J Psychiatry (Vol 212(3). https://doi.org/10.1192/bjp.2017.27
Dachew BA, Scott JG, Mamun A, Alati R (2019) Pre-eclampsia and the risk of attention-deficit/hyperactivity disorder in offspring: findings from the ALSPAC birth cohort study. Psychiatry Res 272. https://doi.org/10.1016/j.psychres.2018.12.123
Ding X, Yang Z, Han Y, Yu H (2015) Correlation of long-chain fatty acid oxidation with oxidative stress and inflammation in pre-eclampsia-like mouse models. Placenta 36(12). https://doi.org/10.1016/j.placenta.2015.10.014
Eide MG, Moster D, Irgens LM, Reichborn-Kjennerud T, Stoltenberg C, Skjærven R, Susser E, Abel K (2013) Degree of fetal growth restriction associated with schizophrenia risk in a national cohort. Psychol Med 43(10). https://doi.org/10.1017/S003329171200267X
Enayati M, Mosaferi B, Homberg JR, Diniz DM, Salari AA (2020) Prenatal maternal stress alters depression-related symptoms in a strain - and sex-dependent manner in rodent offspring. Life Sciences, 251. https://doi.org/10.1016/j.lfs.2020.117597
Fajersztajn L, Veras MM (2017) Hypoxia: From Placental Development to Fetal Programming. In Birth Defects Research (Vol. 109, Issue 17). https://doi.org/10.1002/bdr2.1142
Fraser A, Nelson SM, MacDonald-Wallis C, Sattar N, Lawlor DA (2013) Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension 62(3). https://doi.org/10.1161/HYPERTENSIONAHA.113.01513
Galbally M, Watson SJ, Lappas M, de Kloet ER, Wyrwoll CS, Mark PJ, Lewis AJ (2022) Exploring sex differences in fetal programming for childhood emotional disorders. Psychoneuroendocrinology, 141. https://doi.org/10.1016/j.psyneuen.2022.105764
Gumusoglu SB, Chilukuri ASS, Hing BWQ, Scroggins SM, Kundu S, Sandgren JA, Santillan MK, Santillan DA, Grobe JL, Stevens HE (2021) Altered offspring neurodevelopment in an arginine vasopressin preeclampsia model. Translational Psychiatry 11(1). https://doi.org/10.1038/s41398-021-01205-0
Heikura U, Hartikainen AL, Nordström T, Pouta A, Taanila A, Järvelin MR (2013) Maternal hypertensive disorders during pregnancy and mild cognitive limitations in the offspring. Paediatr Perinat Epidemiol 27(2). https://doi.org/10.1111/ppe.12028
Ianosi-Irimie M, Vu HV, Whitbred JM, Pridjian CA Nadig, J. D., Williams MY, Wrenn DC, Pridjian G, Puschett, Puschett JB (2005) A rat model of Preeclampsia. Clin Exp Hypertens 27(8):605–617. https://doi.org/10.1080/10641960500298608
Kajantie E, Eriksson JG, Osmond O, Thornburg K, Barker DJP (2009) Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke 40(4):1176–1180
Kay VR, Rätsep MT, Figueiró-Filho EA, Croy BA (2019) Preeclampsia may influence offspring neuroanatomy and cognitive function: A role for placental growth factor†. In Biology of Reproduction (Vol. 101, Issue 2). https://doi.org/10.1093/biolre/ioz095
Kay VR, Rätsep MT, Cahill LS, Hickman AF, Zavan B Newport, ME, Ellegood J, Laliberte CL, Reynolds JN, Carmeliet P, Tayade C, Sled JG, Croy BA (2018) Effects of placental growth factor deficiency on behavior, neuroanatomy, and cerebrovasculature of mice. Physiol Genom 50(10). https://doi.org/10.1152/physiolgenomics.00076.2018
Laloux C, Mairesse J, Van Camp G, Giovine A, Branchi I, Bouret S, Morley-Fletcher S, Bergonzelli G, Malagodi M, Gradini R, Nicoletti F, Darnaudéry M, Maccari S (2012) Anxiety-like behaviour and associated neurochemical and endocrinological alterations in male pups exposed to prenatal stress. Psychoneuroendocrinology 37(10). https://doi.org/10.1016/j.psyneuen.2012.02.010
Laviola G, Macrì S, Morley-Fletcher S, Adriani W (2003) Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev 27(1–2). https://doi.org/10.1016/S0149-7634(03)00006-X
Liu X, Zhao W, Liu H, Kang Y, Ye C, Gu W, Hu R, Li X (2016) Developmental and functional brain impairment in offspring from Preeclampsia-Like rats. Mol Neurobiol 53(2). https://doi.org/10.1007/s12035-014-9060-7
Liu L, Lin Z, Zheng B, Wang L, Zou J, Wu S, Jiang Z, Jin Q, Lai X, Lin P (2020) Reduced intellectual ability in offspring born from preeclamptic mothers: A prospective cohort study. Risk Management and Healthcare Policy, 13. https://doi.org/10.2147/RMHP.S277521
Longtine MS, Nelson DM (2011) Placental dysfunction and fetal programming: the importance of placental size, shape, histopathology, and molecular composition. Semin Reprod Med 29(3). https://doi.org/10.1055/s-0031-1275515
Lu SF, Lin QF, Li Y, Jiang XJ (2019) Synthesis of nomega-Nitro-L-arginine methyl ester modified reduced graphene oxide nanosheets and their protective action on experimental preeclampsia in mice. J Photochem Photobiol B 194. https://doi.org/10.1016/j.jphotobiol.2019.03.013
Maher GM, Dalman C, O’Keeffe GW, Kearney PM, McCarthy FP, Kenny LC, Khashan AS (2020) Association between preeclampsia and attention-deficit hyperactivity disorder: a population-based and sibling-matched cohort study. Acta Psychiatrica Scandinavica 142(4). https://doi.org/10.1111/acps.13162
Mann JR, McDermott S, Bao H, Hardin J, Gregg A (2010) Pre-eclampsia, birth weight, and autism spectrum disorders. J Autism Dev Disord 40(5). https://doi.org/10.1007/s10803-009-0903-4
Motta C, Grosso C, Zanuzzi C, Molinero D, Picco N, Bellingeri R, Alustiza F, Barbeito C, Vivas A, Romanini MC (2015) Effect of Sildenafil on Pre-eclampsia-like Mouse Model Induced by L-Name. Reprod Domest Anim 50(4). https://doi.org/10.1111/rda.12536
Moura CA, Oliveira MC, Costa LF, Tiago PRF, Holanda VAD, Lima RH, Cagni FC, Lobão-Soares B, Bolaños-Jiménez F, Gavioli EC (2020) Prenatal Restraint stress impairs Recognition Memory in Adult Male and female offspring. Acta Neuropsychiatrica. https://doi.org/10.1017/neu.2020.3
Phipps EA, Thadhani R, Benzing T, Karumanchi SA (2019) Pre-eclampsia: pathogenesis, novel diagnostics and therapies. In Nature Reviews Nephrology (Vol. 15, Issue 5). https://doi.org/10.1038/s41581-019-0119-6
Ramírez-Vélez R (2012) Programación Fetal in utero y su impacto en la salud del adulto. In Endocrinologia y Nutricion (Vol. 59, Issue 6). https://doi.org/10.1016/j.endonu.2012.02.002
Rätsep MT, Hickman AF, Maser B, Pudwell J, Smith GN, Brien D, Stroman PW, Adams MA, Reynolds JN, Croy BA, Paolozza A (2016) Impact of preeclampsia on cognitive function in the offspring. Behavioural Brain Research, 302. https://doi.org/10.1016/j.bbr.2016.01.030
Reynolds LP, Borowicz PP, Caton JS, Crouse MS, Dahlen CR, Ward AK (2019) Developmental Programming of Fetal Growth and Development. In Veterinary Clinics of North America - Food Animal Practice (Vol. 35, Issue 2). https://doi.org/10.1016/j.cvfa.2019.02.006
Rosa MJ, Nentin F, Bosquet Enlow M, Hacker MR, Pollas N, Coull B, Wright RJ (2019) Sex-specific associations between prenatal negative life events and birth outcomes. Stress 22(6). https://doi.org/10.1080/10253890.2019.1608944
Salari AA, Fatehi-Gharehlar L, Motayagheni N, Homberg JR (2016) Fluoxetine normalizes the effects of prenatal maternal stress on depression- and anxiety-like behaviors in mouse dams and male offspring. Behav Brain Res 311. https://doi.org/10.1016/j.bbr.2016.05.062
Shah R, Courtiol E, Castellanos FX, Teixeira CM (2018) Abnormal serotonin levels during perinatal development lead to behavioral deficits in adulthood. Front Behav Neurosci 12. https://doi.org/10.3389/fnbeh.2018.00114
Silva D, Colvin L, Hagemann E, Bower C (2014) Environmental risk factors by gender associated with attention-deficit/ hyperactivity disorder. Pediatrics 133(1). https://doi.org/10.1542/peds.2013-1434
Sørensen HJ, Mortensen EL, Reinisch JM, Mednick SA (2003) Do hypertension and diuretic treatment in pregnancy increase the risk of schizophrenia in offspring? Am J Psychiatry 160(3). https://doi.org/10.1176/appi.ajp.160.3.464
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85(3). https://doi.org/10.1007/BF00428203
Stevenson K, Lillycrop KA, Silver MJ (2020) Fetal programming and epigenetics. In Current Opinion in Endocrine and Metabolic Research (Vol. 13). https://doi.org/10.1016/j.coemr.2020.07.005
Sverrisson FA, Bateman BT, Aspelund T, Skulason S, Zoega H (2018) Preeclampsia and academic performance in children: a nationwide study from Iceland. PLoS ONE 13(11). https://doi.org/10.1371/journal.pone.0207884
Tuovinen S, Aalto-Viljakainen T, Eriksson JG, Kajantie E, Lahti J, Pesonen AK, Heinonen K, Lahti M, Osmond C, Barker DJP, Räikkönen K (2014) Maternal hypertensive disorders during pregnancy: adaptive functioning and psychiatric and psychological problems of the older offspring. BJOG: Int J Obstet Gynecol 121(12). https://doi.org/10.1111/1471-0528.12753
Van den Hove DLA, Leibold NK, Strackx E, Martinez-Claros M, Lesch KP, Steinbusch HWM, Schruers KRJ, Prickaerts J (2014) Prenatal stress and subsequent exposure to chronic mild stress in rats; interdependent effects on emotional behavior and the serotonergic system. Eur Neuropsychopharmacol 24(4). https://doi.org/10.1016/j.euroneuro.2013.09.006
Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2(2). https://doi.org/10.1038/nprot.2007.44
Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I (2015) Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr 169(2). https://doi.org/10.1001/jamapediatrics.2014.2645
Zuena AR, Mairesse J, Casolini P, Cinque C, Alemà GS, Morley-Fletcher S, Chiodi V, Spagnoli LG, Gradini R, Catalani A, Nicoletti F, Maccari S (2008) Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS ONE 3(5). https://doi.org/10.1371/journal.pone.0002170
Acknowledgements
The authors would like to thank to Beatriz Cruz Lopez for her excellent technical assistance. We acknowledge Mrs. Gena Ingelido’s help for her excellent assistance in English language revision. This study was supported by projects SIP-Instituto Politécnico Nacional, México (20230781 and 20231264) and Instituto Nacional de Psiquiatría “Ramón de la Fuente Muñiz”, México.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vélez-Godínez, B.R., López-Sánchez, P. & Páez-Martínez, N. Preeclampsia as a possible risk factor for memory impairment, anxiety- and depressive-like behavior in offspring. Psychopharmacology (2024). https://doi.org/10.1007/s00213-024-06568-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00213-024-06568-y