Abstract
Rationale
Using routine synthetic drugs in the treatment of psychiatric disorders may have some restrictions due to serious side effects and pharmacoresistance. Some natural agents may be promising alternatives in this case. The neuroprotective activity of the neuromodulator adenosine and its receptor, A1 receptor (A1R) in the central nervous system has been mentioned in different studies.
Objective
We aimed to determine the anxiolytic, antidepressant and sedative effects of Japanese sake yeast as the first report.
Method
Mice were subjected to a one-week stress protocol and concomitantly treated orally with sake yeast at the dose levels of 100, 200 and 300 mg kg-1 once daily for a week. The anxiolytic, antidepressant, and sedative actions of sake yeast were evaluated with the related tests.
Results
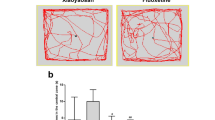
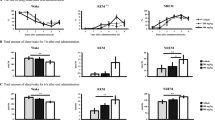
In all dose regiments, sake yeast significantly improved functions in the EPM and FST. 200 and 300 mg/kg of sake yeast significantly increased sleep duration and reduced sleep latency. Anxiolytic and antidepressant-like activities of sake yeast were maintained by the injection of ZM241385 (15 mg kg−1), a selective adenosine A2AR antagonist but completely counteracted by the injection of 8-cyclopentyltheophylline (10 mg kg−1), a selective adenosine A1R antagonist. 300 mg/kg of the yeast significantly increased the BDNF levels. Amygdala corticosterone levels did not show any significant changes at any dosage. Amygdala TNF-α, IL-6 and IL-1β levels also decreased significantly with all the sake regiments compared to the control group.
Conclusions
We conclude that oral sake yeast supplement exerts a neurobehavioral protective effect predominantly by activating central A1Rs.







Similar content being viewed by others
References
Ambavade SD, Mhetre NA, Tate VD, Bodhankar SL (2006) Pharmacological evaluation of the extracts of Sphaeranthus indicus flowers on anxiolytic activity in mice. Indian J Pharmacol 38:254–259
Bhattacharya A, Derecki NC, Lovenberg TW, Drevets WC (2016) Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology 233:1623–1636
Biber K, Lubrich B, Fiebich BL, Boddeke HW, van Calker D (2001) Interleukin-6 enhances expression of adenosine A1 receptor mRNA and signaling in cultured rat cortical astrocytes and brain slices. Neuropsychopharmacology 24:86–96
Biber K, Pinto-Duarte A, Wittendorp MC, Dolga AM, Fernandes CC, Von Frijtag Drabbe Künzel J et al (2008) Interleukin-6 upregulates neuronal adenosine A1 receptors: implications for neuromodulation and neuroprotection. Neuropsychopharmacology 33:2237–2250
Borsini F, Podhorna J, Marazziti D (2002) Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 163:121–141
Cunha RA (2005) Neuroprotection by adenosine in the brain: From A1 receptor activation to A2A receptor blockade. Purinergic Signall 1:111–134
Deutschenbaur L, Beck J, Kiyhankhadiv A, Mühlhauser M, Borgwardt S, Walter M et al (2016) Role of calcium, glutamate and NMDA in major depression and therapeutic application. Prog Neuro-Psychopharmacol Biol Psychiat 64:325–333
Guieu R, Dussol B, Halimi G, Bechis G, Sampieri F, Berland Y et al (1998) Adenosine and the nervous system: Pharmacological data and therapeutic perspectives. Gen Pharmacol 31:553–561
Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M (2015) Cumulative meta-analysis of interleukins 6 and 1beta, tumor necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behavior Immun 49:206–215
Jeon SJ, Rhee SY, Ryu JH, Cheong JH, Kwon K, Yang SI et al (2011) Activation of adenosine A2A receptor up-regulates BDNF expression in rat primary cortical neurons. Neurochem Res 36:2259–2269
Kakeda S, Watanabe K, Katsuki A, Sugimoto K, Igata N, Ueda I et al (2018) Relationship between interleukin (IL)-6 and brain morphology in drug-naive, first-episode major depressive disorder using surface-based morphometry. Scientific Reports 8:10054
Kent JM, Coplan JD, Gorman JM (1998) Clinical utility of the selective serotonin reuptake inhibitors in the spectrum of anxiety. Biol Psychiat 44:812–824
Köhler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N et al (2018) Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol 55:4195–4206
Kulesskaya N, Voikar V (2014) Assessment of mouse anxiety-like behavior in the light-dark box and openfield arena: role of equipment and procedure. Physiol Behavior 133:30–38
McLeod DR, Hoehn-Saric R, Zimmerli WD, De Souza EB, Oliver LK (1990) Treatment effects of alprazolam and imipramine: physiological versus subjective changes in patients with generalized anxiety disorder. Biol Psychiat 28:849–861
Moidunny S, Dias RB, Wesseling E, Sekino Y, Boddeke HW, Sebastiao AM et al (2010) Interleukin-6- type cytokines in neuroprotection and neuromodulation: Oncostatin M, but not leukemia inhibitory factor, requires neuronal adenosine A1 receptor function. J Neurochem 114:1667–1677
Monoi N, Matsuno A, Nagamori Y, Kimura E, Nakamura Y, Oka K et al (2016) Japanese sake yeast supplementation improves the quality of sleep: a double-blind randomized controlled clinical trial. J Sleep Res 25:116–123
Nakahara M, Mishima T, Hayakawa T (2007) Effect of a sake concentrate on the epidermis of aged mice and confirmation of ethyl α-D-glucoside as its active component. Biosci Biotechnol Biochem 71:427–434
Nakamura Y, Midorikawa T, Monoi N, Kimura E, Murata-Matsuno A, Sano T et al (2016) Oral administration of Japanese sake yeast (Saccharomyces cerevisiae sake) promotes non-rapid eye movement sleep in mice via adenosine A2A receptors. J Sleep Res 25:746–753
Palmer TM, Stiles GL (1995) Adenosine receptors. Neuropharmacology 34:683–694
Paul IA, Skolnick P (2003) Glutamate and depression: clinical and preclinical studies. Annals New York Acad Sci 1003:250–272
Peirce JM, Alviña K (2019) The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res 97:1223–1241
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open: Closed arm entries in an elevated plus maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Phillis JW, Wu PH (1982) Adenosine mediates sedative action of various centrally active drugs. Med Hypotheses 9:361–367
Porsolt RD, Bertin A, Jalfre M (1977) Behavioural despair in mice: a primary screening test for antidepressants. Archives Internationales de Pharmacodynamie et de Therapie 229:327–336
Prediger RDS, Batista LC, Takahashi RN (2004) Adenosine A1 receptors modulate the anxiolytic-like effect of ethanol in the elevated plus-maze in mice. Eur J Pharmacol 499:147–154
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol 463:3–33
Ramirez K, Sheridan JF (2016) Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive- like behaviors. Brain Behavior Immun 57:293–303
Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB (1992) Neuroprotective role of adenosine in cerebral ischemia. Trends Pharmacol Sci 13:439–445
Sebastião AM, Assaife-Lopes N, Diógenes MJ, Vaz SH, Ribeiro JA (2011) Modulation of brain-derived neurotrophic factor (BDNF) actions in the nervous system by adenosine A2A receptors and the role of lipid rafts. Biochimica et Biophysica Acta (BBA) - Biomembranes 1808:1340–1349
Serchov T, Clement HW, Schwarz MK, Iasevoli F, Tosh DK, Marco I et al (2015) Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of Homer1a. Neuron 87:549–562
Slusarczyk J, Trojan E, Chwastek J, Glombik K, Basta-Kaim A (2016) A potential contribution of chemokine network dysfunction to the depressive disorders. Curr Neuropharmacol 14:705–720
Stockwell J, Jakova E, Cayabyab FS (2017) Adenosine A1 and A2A receptors in the brain: current research and their role in neurodegeneration. Molecules 22:E676
Tsutsui S, Schnermann J, Noorbakhsh F, Henry H, Yong VW, Winston BW et al (2004) A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J Neurosci 24:1521–1529
van Calker D, Biber K, Domschke K, Serchov T (2019) The role of adenosine receptors in mood and anxiety disorders. J Neurochem 51:11–27
Vásquez CE, Riener R, Reynolds E, Britton GB (2014) NMDA receptor dysregulation in chronic state: a possible mechanism underlying depression with BDNF downregulation. Neurochem Int 79:88–97
Vazquez JF, Clement HW, Sommer O, Schulz E, van Calker D (2008) Local stimulation of the adenosine A2B receptors induces an increased release of IL-6 in mouse striatum: an in vivo microdialysis study. J Neurochem 105:904–909
Vincenzi F, Borea PA, Varani K (2017) Anxiolytic properties of A1 adenosine receptor PAMs. Oncotarget 318:7216–7217
Wohleb ES, Franklin T, Iwata M, Duman RS (2016) Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci 17:497–511
Yamada K, Kobayashi M, Mori A, Jenner P, Kanda T (2013) Antidepressant-like activity of the adenosine A(2A) receptor antagonist, istradefylline (KW-6002), in the forced swim test and the tail suspension test in rodents. Pharmacol Biochem Behavior 114-115:23–30
Yamada K, Kobayashi M, Kanda T (2014) Involvement of adenosine A2A receptors in depression and anxiety. Int Rev Neurobiol 119:373–393
Zhan Y, Xia J, Wang X (2020) Effects of glutamate-related drugs on anxiety and compulsive behavior in rats with obsessive-compulsive disorder. Int J Neurosci 130:551–560
Zhang C, Zhao X, Mao X, Liu A, Liu Z, Li X et al (2014) Pharmacological evaluation of sedative and hypnotic effects of schizandrin through the modification of pentobarbital-induced sleep behaviors in mice. Eur J Pharmacol 744:157–163
The authors are grateful to the Iran National Science Foundation (INSF) and the Research Center of Physiology of Semnan University of Medical Sciences for their cooperation and also Prof. Dr. Thomas Budde for providing the supplement (medicine) for the present study.
Funding statement
This study was funded by Iran National Science Foundation (INSF), Tehran, Iran [grant number 98012571].
Data availabilitty statement
Raw data were originated from the Research Center of Physiology, Semnan University of Medical Sciences, Semnan. Supporting and analyzed data for results of this study are available from the corresponding authors Nasrollah Moradikor or Ali Rashidy-Pour on request.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval statement
The experiments of the present study were approved by the Ethical Reviews Committee of Semnan University of Medical Sciences and Health Services (approval ID: IR.SEMUMS.REC.1398.275).
Conflicts of interest disclosure
The authors declare that there are no competing interests in this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bozorgi, H., Rashidy-Pour, A., Moradikor, N. et al. Neurobehavioral protective effects of Japanese sake yeast supplement against chronic stress-induced anxiety and depression-like symptoms in mice: Possible role of central adenosine receptors. Psychopharmacology 241, 401–416 (2024). https://doi.org/10.1007/s00213-023-06496-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06496-3