Abstract
Background
Ethanol use disorders are a serious medical and public health problem in the world today. Acute ethanol intoxication can lead to cognitive dysfunction such as learning and memory impairment. Gamma oscillations (γ, 30–80 Hz) are synchronized rhythmic activity generated by population of neurons within local network, and closely related to learning and memory function. The hippocampus is a critical anatomic structure that supports learning and memory. On the grounds of structure and function, hippocampus can be divided into the intermediate (IH), the dorsal (DH), and ventral hippocampus (VH). The current study is the first to investigate the effects of acute ethanol on γ oscillations in these sub-regions of rat hippocampal slices.
Methods
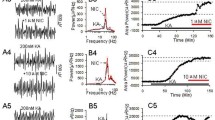
The sustained γ oscillations were induced by 200 nM kainate (KA) in the CA3c of IH, DH, and VH. When KA-induced γ oscillation reached the steady state, ethanol (50 mM or 100 mM) was applied and the effects of ethanol on γ oscillation power was measured in the slices sequentially sectioned from ventral to dorsal hippocampus of adult rats.
Results
In the intermediate hippocampal slices, compared with control (KA only), ethanol (50 mM) caused 36.1 ± 3.9% decrease in γ power (p < 0.05, n = 10), while ethanol (100 mM) caused 55.3 ± 5.5% decrease in γ power (p < 0.001, n = 14). In the dorsal hippocampus, only ethanol (100 mM) caused 18.1 ± 8.6% decrease in γ power (p < 0.05, n = 12). However, in the ventral hippocampus, neither 50 mM nor 100 mM ethanol affected γ oscillation.
Conclusions
Our results demonstrate that ethanol may produce the differential suppression of γ oscillations in a dose-dependent manner in different sub-regions of hippocampus, suggesting that the modulation of ethanol on hippocampal γ oscillation is region-dependent.




Similar content being viewed by others
References
Ariwodola OJ, Weiner JL (2004) Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci 24(47):10679–10686
Bast T (2007) Toward an integrative perspective on hippocampal function: from the rapid encoding of experience to adaptive behavior. Rev Neurosci 18:253–282
Bast T, Wilson IA, Witter MP, Morris RGM (2009) From rapid place learning to behavioral performance: a key role for the intermediate hippocampus. PLoS Biol 7(4):e1000089
Blitzer RD, Gil O, Landau EM (1990) Long-term potentiation in rat hippocampus is inhibited by low concentrations of ethanol. Brain Res 537(1–2):203–208
Bocarsly ME, da Silva e Silva D, Kolb V, Luderman KD, Shashikiran S, Rubinstein M, Sibley DR, Dobbs LK, Alvarez VA (2019) A mechanism linking two known vulnerability factors for alcohol abuse: heightened alcohol stimulation and low striatal dopamine D2 receptors. Cell Rep 29(5):1147–1163.e5
Burwell RD (2000) The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci 911:25–42
Campbell AE, Sumner P, Singh KD, Muthukumaraswamy SD (2014) Acute effects of alcohol on stimulus-induced gamma oscillations in human primary visual and motor cortices. Neuropsychopharmacology 39(9):2104–2113
Cenquizca LA, Swanson LW (2007) Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res Rev 56(1):1–26
Colgin LL (2016) Rhythms of the hippocampal network. Nat Rev Neurosci 17(4):239–249
Coller JK, Hutchinson MR (2012) Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther 134(2):219–245
Dubovyk V, Manahan-Vaughan D (2019) Gradient of expression of dopamine D2 receptors along the dorso-ventral axis of the hippocampus. Front Synaptic Neurosci 11:28
Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65(1):7–19
Ferrani-Kile K, Randall PK, Leslie SW (2003) Acute ethanol affects phosphorylation state of the NMDA receptor complex: implication of tyrosine phosphatases and protein kinase a. Brain Res Mol Brain Res 115(1):78–86
Flentke GR, Garic A, Hernandez M, Smith SM (2014) CaMKII represses transcriptionally active beta-catenin to mediate acute ethanol neurodegeneration and can phosphorylate beta-catenin. J Neurochem 128(4):523–535
Fries P, Nikolic D, Singer W (2007) The gamma cycle. Trends Neurosci 30(7):309–316
Fuster-Matanzo A et al (2011) Different susceptibility to neurodegeneration of dorsal and ventral hippocampal dentate gyrus: a atudy with transgenic mice overexpressing GSK3β. PLoS One 6(11):e27262
Harper C (1998) The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol 57(2):101–110
Hoffmann SE, Matthews DB (2001) Ethanol-induced impairments in spatial working memory are not due to deficits in learning. Alcohol Clin Exp Res 25(6):856–861
Ishizuka N (2001) Laminar organization of the pyramidal cell layer of the subiculum in the rat. J Comp Neurol 435(1):89–110
Izumi Y, Nagashima K, Murayama K, Zorumski CF (2005) Acute effects of ethanol on hippocampal long-term potentiation and long-term depression are mediated by different mechanisms. Neuroscience 136(2):509–517
Jiang H et al (2020) Distinct directional couplings between slow and fast gamma power to the phase of theta oscillations in the rat hippocampus. Eur J Neurosci 51(10):2070–2081
Kemp A, Manahan-Vaughan D (2007) Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci 30(3):111–118
Kenney J, Manahan-Vaughan D (2013) Learning-facilitated synaptic plasticity occurs in the intermediate hippocampus in association with spatial learning. Front Synaptic Neurosci 5:10
Kishi T, Tsumori T, Yokota S, Yasui Y (2006) Topographical projection from the hippocampal formation to the amygdala: a combined anterograde and retrograde tracing study in the rat. J Comp Neurol 496(3):349–368
Lovinger DM (2002) NMDA receptors lose their inhibitions. Nat Neurosci 5(7):614–616
Lovinger DM, White G, Weight FF (1990) NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci 10(4):1372–1379
Lu CB, Jefferys JGR, Toescu EC, Vreugdenhil M (2011) In vitro hippocampal gamma oscillation power as an index of in vivo CA3 gamma oscillation strength and spatial reference memory. Neurobiol Learn Mem 95(3):221–230
Matta R, Tiessen A, Choleris E (2017) The role of dorsal hippocampal dopamine D1-type receptors in social learning, social interactions, and food intake in male and female mice. Neuropsychopharmacology 42(12):2344–2353
Matthews DB, Silvers JR (2004) The use of acute ethanol administration as a tool to investigate multiple memory systems. Neurobiol Learn Mem 82(3):299–308
Moonat S, Starkman BG, Sakharkar A, Pandey SC (2010) Neuroscience of alcoholism: molecular and cellular mechanisms. Cell Mol Life Sci 67(1):73–88
Moselhy HF, Georgiou G, Kahn A (2001) Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol 36(5):357–368
Pandis C, Sotiriou E, Kouvaras E, Asprodini E, Papatheodoropoulos C, Angelatou F (2006) Differential expression of NMDA and AMPA receptor subunits in rat dorsal and ventral hippocampus. Neuroscience 140(1):163–175
Petrovich GD, Canteras NS, Swanson LW (2001) Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev 38(1–2):247–289
Pitkanen A et al (2000) Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci 911:369–391
Proctor WR, Diao L, Freund RK, Browning MD, Wu PH (2006) Synaptic GABAergic and glutamatergic mechanisms underlying alcohol sensitivity in mouse hippocampal neurons. J Physiol 575(Pt 1):145–159
Rabin RA, Edelman AM, Wagner JA (1992) Activation of protein kinase A is necessary but not sufficient for ethanol-induced desensitization of cyclic AMP production. J Pharmacol Exp Ther 262(1):257–262
Risold PY, Thompson RH, Swanson LW (1997) The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Brain Res Rev 24(2–3):197–254
Taube JS (2007) The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci 30:181–207
Traub RD, Bibbig A, LeBeau FEN, Buhl EH, Whittington MA (2004) Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci 27:247–278
Van Groen T, Wyss JM (2003) Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol 463(3):249–263
Wang J et al (2016) Acute ethanol inhibition of γ oscillations is mediated by Akt and GSK3β. Front Cell Neurosci 10:189
Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bahner F, Both M, Tort ABL, Kopell NJ, Wisden W, Monyer H (2009) Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A 106(9):3561–3566
Xu M, Woodward JJ (2006) Ethanol inhibition of NMDA receptors under conditions of altered protein kinase a activity. J Neurochem 96(6):1760–1767
Zeng T, Zhang CL, Song FY, Zhao XL, Yu LH, Zhu ZP, Xie KQ (2012) PI3K/Akt pathway activation was involved in acute ethanol-induced fatty liver in mice. Toxicology 296(1):56–66
Funding
This study was supported by the National Natural Science Foundation of China (NSFC, grant numbers: 81271422; U1804170; 81100953; 1070938), International Collaboration of Henan Province Science-Technique Bureau (134300510040), Natural Science Foundation of Hebei Province (H2012203067) and Key Program for Applied Basic Research of Hebei Key Program for Applied Basic Research of Hebei Province (12966119D).
Author information
Authors and Affiliations
Contributions
ZRL and LCB designed and supervised the experiments; LZH, YB, and GFL performed the experiments; RF, ZHX, ZXY, LZH, and LCB analyzed the data. LCB, LZH, and ZRL wrote and revised the manuscript; All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Z., Ren, F., Yang, B. et al. Region-dependent regulation of acute ethanol on γ oscillation in the rat hippocampal slices. Psychopharmacology 237, 2959–2966 (2020). https://doi.org/10.1007/s00213-020-05584-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05584-y