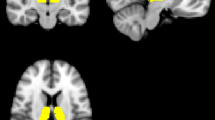
Abstract
Background
The thalamus is a major target of dopaminergic projections and is densely connected with the prefrontal cortex. A better understanding of how dopamine changes thalamo-cortical communication may shed light on how dopamine supports cognitive function. Methylphenidate has been shown to facilitate cognitive processing and reduce connectivity between the thalamus and lateral prefrontal cortex.
Aims
The thalamus is a heterogeneous structure, and the present study sought to clarify how the intrinsic connections of thalamic sub-regions are differentially impacted by acute dopamine transporter blockade.
Methods
Sixty healthy volunteers were orally administered either 20 mg of methylphenidate (N = 29) or placebo (N = 31) in a double-blind, randomized, between-subject design. Multi-echo fMRI was used to assess intrinsic functional connectivity of sub-regions of the thalamus during a resting state scan. An N-back working-memory paradigm provided a measure of cognitive performance.
Results
Acute methylphenidate significantly reduced connectivity of the lateral prefrontal cortex with the motor and somatosensory sub-regions of the thalamus and reduced connectivity with the parietal and visual sub-regions at a trend level. Connectivity with the premotor, prefrontal, and temporal sub-regions was not impacted. The intrinsic connectivity between the thalamus and the lateral prefrontal cortex was not associated with working-memory performance.
Conclusions
Methylphenidate decreases functional connections between the lateral prefrontal cortex and thalamus broadly, while sparing intrinsic connectivity with thalamic sub-regions involved with working-memory and language related processes. Collectively, our results suggest that the dopamine transporter regulates functional connections between the prefrontal cortex and non-cognitive areas of the thalamus.



Similar content being viewed by others
References
Arnsten AF, Scahill L, Findling RL (2007) Alpha-2 adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. J Child Adolesc Psychopharmacol 17(4):393–406
Baddeley A (1992) Working memory. Science 255(5044):556–559. https://doi.org/10.1126/science.1736359
Barbas H, García-Cabezas MÁ, Zikopoulos B (2013) Frontal-thalamic circuits associated with language. Brain Lang 126(1):49–61
Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O (2003) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6(7):750–757
Chen G, Adleman NE, Saad ZS, Leibenluft E, Cox RW (2014) Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. Neuroimage 99:571–588
Collins DP, Anastasiades PG, Marlin JJ, Carter AG (2018) Reciprocal circuits linking the prefrontal cortex with dorsal and ventral thalamic nuclei. Neuron 98(2):366–379
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29(3):162–173
Cox RW, Reynolds RC, Taylor PA (2016) AFNI and clustering: false positive rates redux. BioRxiv 065862
de Bourbon-Teles J, Bentley P, Koshino S, Shah K, Dutta A, Malhotra P, Egner T, Husain M, Soto D (2014) Thalamic control of human attention driven by memory and learning. Curr Biol 24(9):993–999
Demiral ŞB, Tomasi D, Wiers CE, Manza P, Shokri-Kojori E, Studentsova Y, Wang G-J, Volkow ND (2018) Methylphenidate’s effects on thalamic metabolism and functional connectivity in cannabis abusers and healthy controls. Neuropsychopharmacology 1
Ding Y-S, Fowler JS, Volkow ND, Dewey SL, Wang G-J, Logan J, Gatley SJ, Pappas N (1997) Chiral drugs: comparison of the pharmacokinetics of [11C] d-threo and L-threo-methylphenidate in the human and baboon brain. Psychopharmacology 131(1):71–78
Eklund A, Nichols TE, Knutsson H (2016) Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci 113(28):7900–7905
Ernst M, Lago T, Davis A, Grillon C (2016) The effects of methylphenidate and propranolol on the interplay between induced-anxiety and working memory. Psychopharmacology 233(19):3565–3574. https://doi.org/10.1007/s00213-016-4390-y
Farr OM, Zhang S, Hu S, Matuskey D, Abdelghany O, Malison RT, Li CR (2014) The effects of methylphenidate on resting-state striatal, thalamic and global functional connectivity in healthy adults. Int J Neuropsychopharmacol 17(8):1177–1191
First MB, Spitzer RL, Gibbon M, Williams JB (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edn. SCID-I/P, New York
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33(3):341–355
García-Cabezas MÁ, Rico B, Sánchez-González MÁ, Cavada C (2007) Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage 34(3):965–984
Hagoort P (2014) Nodes and networks in the neural architecture for language: Broca’s region and beyond. Curr Opin Neurobiol 28:136–141
Hannestad J, Gallezot J-D, Planeta-Wilson B, Lin S-F, Williams WA, van Dyck CH, Malison RT, Carson RE, Ding Y-S (2010) Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry 68(9):854–860
Hauser TU, Fiore VG, Moutoussis M, Dolan RJ (2016) Computational psychiatry of ADHD: neural gain impairments across Marrian levels of analysis. Trends Neurosci 39(2):63–73
Huang Q, Zhou D, Chase K, Gusella JF, Aronin N, DiFiglia M (1992) Immunohistochemical localization of the D1 dopamine receptor in rat brain reveals its axonal transport, pre- and postsynaptic localization, and prevalence in the basal ganglia, limbic system, and thalamic reticular nucleus. Proc Natl Acad Sci 89(24):11988–11992
Iglesias JE, Insausti R, Lerma-Usabiaga G, Bocchetta M, Van Leemput K, Greve DN, van der Kouwe A, Fischl B, Caballero-Gaudes C, Paz-Alonso PM (2018) A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. NeuroImage 183:314–326. https://doi.org/10.1016/j.neuroimage.2018.08.012
Jacob SN, Nieder A (2014) Complementary roles for primate frontal and parietal cortex in guarding working memory from distractor stimuli. Neuron 83(1):226–237. https://doi.org/10.1016/j.neuron.2014.05.009
Koelsch S, Schulze K, Sammler D, Fritz T, Müller K, Gruber O (2009) Functional architecture of verbal and tonal working memory: an FMRI study. Hum Brain Mapp 30(3):859–873
Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ (2013) Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA Psychiatry 70(8):857–868
Konova AB, Moeller SJ, Tomasi D, Goldstein RZ (2015) Effects of chronic and acute stimulants on brain functional connectivity hubs. Brain Res 1628:147–156
Kundu P, Voon V, Balchandani P, Lombardo MV, Poser BA, Bandettini PA (2017) Multi-echo fMRI: a review of applications in fMRI denoising and analysis of BOLD signals. Neuroimage 154:59–80
Kupferschmidt DA, Gordon JA (2018) The dynamics of disordered dialogue: prefrontal, hippocampal and thalamic miscommunication underlying working memory deficits in schizophrenia. Brain Neurosci Adv 2:2398212818771821
Linssen AMW, Vuurman E, Sambeth A, Riedel WJ (2012) Methylphenidate produces selective enhancement of declarative memory consolidation in healthy volunteers. Psychopharmacology 221(4):611–619
Mitchell AS (2015) The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making. Neurosci Biobehav Rev 54:76–88. https://doi.org/10.1016/j.neubiorev.2015.03.001
Mueller S, Costa A, Keeser D, Pogarell O, Berman A, Coates U, Reiser MF, Riedel M, Möller H-J, Ettinger U (2014) The effects of methylphenidate on whole brain intrinsic functional connectivity. Hum Brain Mapp 35(11):5379–5388
Owen AM, Stern CE, Look RB, Tracey I, Rosen BR, Petrides M (1998) Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proc Natl Acad Sci 95(13):7721–7726. https://doi.org/10.1073/pnas.95.13.7721
Parnaudeau S, O’Neill P-K, Bolkan SS, Ward RD, Abbas AI, Roth BL, Balsam PD, Gordon JA, Kellendonk C (2013) Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron 77(6):1151–1162. https://doi.org/10.1016/j.neuron.2013.01.038
Pergola G, Danet L, Pitel A-L, Carlesimo GA, Segobin S, Pariente J, Suchan B, Mitchell AS, Barbeau EJ (2018) The regulatory role of the human mediodorsal thalamus. Trends Cogn Sci
Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014) Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84:320–341
Ramaekers JG, Evers EA, Theunissen EL, Kuypers KPC, Goulas A, Stiers P (2013) Methylphenidate reduces functional connectivity of nucleus accumbens in brain reward circuit. Psychopharmacology 229(2):219–226
Ranganath A, Jacob SN (2016) Doping the mind: dopaminergic modulation of prefrontal cortical cognition. Neuroscientist 22(6):593–603. https://doi.org/10.1177/1073858415602850
Sakai K, Rowe JB, Passingham RE (2002) Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci 5(5):479–484
Sánchez-González MÁ, García-Cabezas MÁ, Rico B, Cavada C (2005) The primate thalamus is a key target for brain dopamine. J Neurosci 25(26):6076–6083
Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, Halassa MM (2017) Thalamic amplification of cortical connectivity sustains attentional control. Nature 545(7653):219–223
Sherman SM (2011) Functioning of circuits connecting thalamus and cortex. Compr Physiol 7(2):713–739
Spencer RC, Devilbiss DM, Berridge CW (2015) The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex. Biol Psychiatry 77(11):940–950
Swanson JM, Volkow ND (2003) Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev 27(7):615–621
Tanaka M (2007) Cognitive signals in the primate motor thalamus predict saccade timing. J Neurosci 27(44):12109–12118. https://doi.org/10.1523/JNEUROSCI.1873-07.2007
Taylor PA, Saad ZS (2013) FATCAT: (an efficient) functional and tractographic connectivity analysis toolbox. Brain Connect 3(5):523–535
Tomasi D, Volkow ND (2012) Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol Psychiatry 71(5):443–450
Volkow ND, Wang G-J, Fowler JS, Gatley SJ, Logan J, Ding Y-S, Hitzemann R, Pappas N (1998) Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatr 155(10):1325–1331
Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K, Halassa MM (2015) Thalamic control of sensory selection in divided attention. Nature 526(7575):705–709
Xiaob D, Barbas H (2004) Circuits through prefrontal cortex, basal ganglia, and ventral anterior nucleus map pathways beyond motor control. Thalamus Relat Syst 2(4):325–343
Zikopoulos B, Barbas H (2006) Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci 26(28):7348–7361
Acknowledgments
This work utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov).
Funding
This work was supported by the Intramural Research Program of the National Institutes of Mental Health, project no. ZIAMH002798 (clinical protocol 02-M-0321, NCT00047853) to CG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 981 kb)
Rights and permissions
About this article
Cite this article
Gorka, A.X., Lago, T.R., Balderston, N. et al. Intrinsic connections between thalamic sub-regions and the lateral prefrontal cortex are differentially impacted by acute methylphenidate. Psychopharmacology 237, 1873–1883 (2020). https://doi.org/10.1007/s00213-020-05505-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05505-z