Abstract
Rationale
The rapid-onset and long-lasting antidepressant properties of ketamine have prompted investigations into a variety of agents that target N-methyl-D-aspartate receptors (NMDARs) for the treatment of major depressive disorder (MDD). According to the literature, ifenprodil (a GluN2B-containing NMDAR antagonist) can potentiate the antidepressant-like effects of certain antidepressant drugs in mice. Here, we report that a single injection of ifenprodil (3 mg/kg, intraperitoneally (i.p.)) was sufficient to provoke rapid antidepressant-like effects in chronic unpredictable mild stress (CUMS) rats. Moreover, ifenprodil activated mTOR signaling and reversed the CUMS-induced elevation of interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in the hippocampus after acute administration. Unfortunately, in our study, ifenprodil had no influence on corticosterone levels in the plasma. Our data indicate that ifenprodil per se might exert antidepressant-like effects by modulating neuroplasticity and inflammatory processes rather than the typical hormonal factors affected by stressors.
Objectives
To explore the potential rapid antidepressant-like effects and mechanisms of ifenprodil, a GluN2B subunit-selective NMDAR antagonist.
Methods
Male Sprague-Dawley rats were used in 3 separate experiments. In experiment 1, we used the forced swim test (FST) and sucrose preference test (SPT) to identify the rapid antidepressant-like effects of ifenprodil in chronic unpredictable mild stress (CUMS) rats after acute administration. In experiment 2, we assessed neurochemical changes involved in synaptic plasticity within the hippocampus of CUMS rats. In experiment 3, we assessed the levels of corticosterone in the plasma and proinflammatory cytokines in the hippocampus in CUMS rats after ifenprodil treatment.
Results
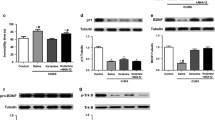
Ifenprodil rapidly ameliorated depressive-like behaviors in the FST and SPT, activated mTOR signaling, dephosphorylated eukaryotic elongation factor 2, enhanced BDNF expression, and promoted the synthesis of the synaptic protein GluA1 synthesis after acute administration. Moreover, ifenprodil reversed the CUMS-induced elevation of interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) in the hippocampus after acute administration. Unfortunately, ifenprodil had no influence on corticosterone levels in the plasma in our study.
Conclusions
Our data indicate that ifenprodil per se might exert antidepressant-like effects through its effects on neuroplasticity and inflammatory processes rather than the typical hormonal factors affected by stressors.





Similar content being viewed by others
References
Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH (2016) Ketamine's mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety 33:689–697
Alexander SP, Mathie A, Peters JA (2008) Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol 153(Suppl 2):S1–S209
Archer S, Chrenek C, Swainson J (2018) Maintenance ketamine therapy for treatment-resistant depression. J Clin Psychopharmacol 38:380–384
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P-f, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95
Baez MV, Cercato MC, Jerusalinsky DA (2018) NMDA receptor subunits change after synaptic plasticity induction and learning and memory acquisition. Neural plasticity 2018:5093048
Banasr M, Duman RS (2008) Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry 64:863–870
Bao AM, Swaab DF (2019) The human hypothalamus in mood disorders: the HPA axis in the center. IBRO reports 6:45–53
Beste C, Stock AK, Ness V, Hoffmann R, Saft C (2015) Evidence for divergent effects of neurodegeneration in Huntington’s disease on attentional selection and neural plasticity: implications for excitotoxicity. Brain Struct Funct 220:1437–1447
Bhattacharyya M, Nandanoor A, Osman M, Kasinathan C, Frederikse P (2014) NMDA glutamate receptor NR1, NR2A and NR2B expression and NR2B Tyr-1472 phosphorylation in the lens. Neurochem Res 39:1825–1832
Brakel K, Hook MA (2019) SCI and depression: does inflammation commandeer the brain? Exp Neurol 320:112977
Cooper MD, Rosenblat JD, Cha DS, Lee Y, Kakar R, McIntyre RS (2017) Strategies to mitigate dissociative and psychotomimetic effects of ketamine in the treatment of major depressive episodes: a narrative review. World J Biol Psychiatry 18:410–423
Coyle CM, Laws KR (2015) The use of ketamine as an antidepressant: a systematic review and meta-analysis. Hum Psychopharmacol 30:152–163
Duman RS, Li N, Liu RJ, Duric V, Aghajanian G (2012) Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 62:35–41
Eric S, Wohleb DG, Thomas A, Duman RS (2017) Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol 15:11–20
Eyre HA, Air T, Pradhan A, Johnston J, Lavretsky H, Stuart MJ, Baune BT (2016) A meta-analysis of chemokines in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry 68:1–8
Fan C, Song Q, Wang P, Li Y, Yang M, Liu B, Yu SY (2018) Curcumin protects against chronic stress-induced dysregulation of neuroplasticity and depression-like behaviors via suppressing IL-1beta pathway in rats. Neuroscience 392:92–106
Fava M, Kendler KS (2000) Major depressive disorder. Neuron 28:335–341
Feder A, Nestler EJ, Charney DS (2009) Psychobiology and molecular genetics of resilience. Nat Rev Neurosci 10:446–457
Frank MG, Thompson BM, Watkins LR, Maier SF (2012) Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun 26:337–345
Freitas AE, Egea J, Buendia I, Gomez-Rangel V, Parada E, Navarro E, Casas AI, Wojnicz A, Ortiz JA, Cuadrado A, Ruiz-Nuno A, Rodrigues ALS, Lopez MG (2016) Agmatine, by improving neuroplasticity markers and inducing Nrf2, prevents corticosterone-induced depressive-like behavior in mice. Mol Neurobiol 53:3030–3045
Garcia LS, Comim CM, Valvassori SS, Reus GZ, Stertz L, Kapczinski F, Gavioli EC, Quevedo J (2009) Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuro-Psychopharmacol Biol Psychiatry 33:450–455
Gerhard DM, Wohleb ES, Duman RS (2016) Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today 21:454–464
Ghasemi M, Montaser-Kouhsari L, Shafaroodi H, Nezami BG, Ebrahimi F, Dehpour AR (2009) NMDA receptor/nitrergic system blockage augments antidepressant-like effects of paroxetine in the mouse forced swimming test. Psychopharmacology 206:325–333
Ghasemi M, Raza M, Dehpour AR (2010) NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. J Psychopharmacol 24:585–594
Gomez-Lazaro E, Arregi A, Beitia G, Vegas O, Azpiroz A, Garmendia L (2011) Individual differences in chronically defeated male mice: behavioral, endocrine, immune, and neurotrophic changes as markers of vulnerability to the effects of stress. Stress (Amsterdam, Netherlands) 14:537–548
Hall BJ, Ghosh A (2008) Regulation of AMPA receptor recruitment at developing synapses. Trends Neurosci 31:82–89
Hall BJ, Ripley B, Ghosh A (2007) NR2B signaling regulates the development of synaptic AMPA receptor current. J Neurosci 27:13446–13456
Hardingham GE, Bading H (2010) Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11:682–696
Harro J (2019) Animal models of depression: pros and cons. Cell Tissue Res 377:5–20
Hashimoto K (2009) Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev 61:105–123
Hashimoto K, Sasaki T, Kishimoto A (2013) Old drug ifenprodil, new hope for PTSD with a history of childhood abuse. Psychopharmacology 227:375–376
Heim C, Binder EB (2012) Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol 233:102–111
Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonte B, Horn S, Lapidus KA, Stelzhammer V, Wong EH, Bahn S, Krishnan V, Bolanos-Guzman CA, Murrough JW, Merad M, Russo SJ (2014) Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A 111:16136–16141
Ji M, Mao M, Li S, Zhang L, Qiu L, Li B, Xia J, Yang J (2019) Acute ketamine administration attenuates lipopolysaccharide-induced depressive-like behavior by reversing abnormal regional homogeneity in the nucleus accumbens. Neuroreport 30:421–427
Jiménez-Sánchez L, Campa L, Auberson YP, Adell A (2014) The role of GluN2A and GluN2B subunits on the effects of NMDA receptor antagonists in modeling schizophrenia and treating refractory depression. Neuropsychopharmacology 39:2673–2680
Kessler RC, Merikangas KR, Wang PS (2007) Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu Rev Clin Psychol 3:137–158
Kishimoto A, Kaneko M, Gotoh Y, Hashimoto K (2012) Ifenprodil for the treatment of flashbacks in female posttraumatic stress disorder patients with a history of childhood sexual abuse. Biol Psychiatry 71:e7–e8
Kohler O, Krogh J, Mors O, Benros ME (2016) Inflammation in depression and the potential for anti-inflammatory treatment. Curr Neuropharmacol 14:732–742
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science (New York, NY) 329:959–964
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761
Li SX, Han Y, Xu LZ, Yuan K, Zhang RX, Sun CY, Xu DF, Yuan M, Deng JH, Meng SQ, Gao XJ, Wen Q, Liu LJ, Zhu WL, Xue YX, Zhao M, Shi J, Lu L (2017) Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects. Mol Psychiatry 23:597–608
Li SX, Han Y, Xu LZ, Yuan K, Zhang RX, Sun CY, Xu DF, Yuan M, Deng JH, Meng SQ, Gao XJ, Wen Q, Liu LJ, Zhu WL, Xue YX, Zhao M, Shi J, Lu L (2018) Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects. Mol Psychiatry 23:597–608
Lin LY, Luo SY, Al-Hawwas M, Herselman MF, Zhou XF, Bobrovskaya L (2019) The long-term effects of ethanol and corticosterone on the mood-related behaviours and the balance between mature BDNF and proBDNF in mice. Journal of molecular neuroscience, MN
Lu Y, Ho CS, Liu X, Chua AN, Wang W, McIntyre RS, Ho RC (2017) Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS One 12:e0186700
Ma XC, Dang YH, Jia M, Ma R, Wang F, Wu J, Gao CG, Hashimoto K (2013) Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice. PLoS One 8:e56053
Marcin LR, Warrier J, Thangathirupathy S, Shi J, Karageorge GN, Pearce BC, Ng A, Park H, Kempson J, Li J, Zhang H, Mathur A, Reddy AB, Nagaraju G, Tonukunuru G, Gupta GVRKM, Kamble M, Mannoori R, Cheruku S, Jogi S, Gulia J, Bastia T, Sanmathi C, Aher J, Kallem R, Srikumar BN, Vijaya KK, Naidu PS, Paschapur M, Kalidindi N, Vikramadithyan R, Ramarao M, Denton R, Molski T, Shields E, Subramanian M, Zhuo X, Nophsker M, Simmermacher J, Sinz M, Albright C, Bristow LJ, Islam I, Bronson JJ, Olson RE, King D, Thompson LA, Macor JE (2018) BMS-986163, a negative allosteric modulator of GluN2B with potential utility in major depressive disorder. ACS Med Chem Lett 9:472–477
McLaughlin KA, Conron KJ, Koenen KC, Gilman SE (2010) Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol Med 40:1647–1658
Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, Hall BJ (2014) GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. eLife 3:e03581
Miller OH, Bruns A, Ben Ammar I, Mueggler T, Hall BJ (2017) Synaptic regulation of a thalamocortical circuit controls depression-related behavior. Cell Rep 20:1867–1880
Muller N (2019) COX-2 inhibitors, aspirin, and other potential anti-inflammatory treatments for psychiatric disorders. Front Psychiatry 10:375
Murrough JW, Abdallah CG, Mathew SJ (2017) Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov 16:472–486
Oliver H, Miller LY, Wang C-C, Hargroder EA, Zhang Y, Delpire E et al (2014) GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. eLife
Page CE, Coutellier L (2018) Adolescent stress disrupts the maturation of anxiety-related behaviors and alters the developmental trajectory of the prefrontal cortex in a sex- and age-specific manner. Neuroscience 390:265–277
Parker KJ, Schatzberg AF, Lyons DM (2003) Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav 43:60–66
Parsons MP, Raymond LA (2014) Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 82:279–293
Pochwat B, Palucha-Poniewiera A, Szewczyk B, Pilc A, Nowak G (2014) NMDA antagonists under investigation for the treatment of major depressive disorder. Expert Opin Investig Drugs 23:1181–1192
Poleszak E, Wosko S, Serefko A, Wlaz A, Kasperek R, Dudka J, Wrobel A, Nowak G, Wlaz P (2014) The effects of ifenprodil on the activity of antidepressant drugs in the forced swim test in mice. Pharmacol Rep 66:1031–1036
Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW (2008) An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28:631–637
Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH (2006) Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral cortex (New York, NY : 1991) 16:313–320
Raison CL, Capuron L, Miller AH (2006) Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27:24–31
Salloum NC, Fava M, Freeman MP, Flynn M, Hoeppner B, Hock RS, Cusin C, Iosifescu DV, Trivedi MH, Sanacora G, Mathew SJ, Debattista C, Ionescu DF, Papakostas GI (2019) Efficacy of intravenous ketamine treatment in anxious versus nonanxious unipolar treatment-resistant depression. Depress Anxiety 36:235–243
Sanacora G (2016) What are we learning from early-phase clinical trials with glutamate targeting medications for the treatment of major depressive disorder. JAMA psychiatry 73:651–652
Schoevers RA, Chaves TV, Balukova SM, aan het Rot M, Kortekaas R (2016) Oral ketamine for the treatment of pain and treatment-resistant depressiondagger. Br J Psychiatry 208:108–113
Sperner-Unterweger B, Kohl C, Fuchs D (2014) Immune changes and neurotransmitters: possible interactions in depression? Prog Neuro-Psychopharmacol Biol Psychiatry 48:268–276
Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S, Dziewczapolski G, Nakamura T, Cao G, Pratt AE, Kang YJ, Tu S, Molokanova E, McKercher SR, Hires SA, Sason H, Stouffer DG, Buczynski MW, Solomon JP, Michael S, Powers ET, Kelly JW, Roberts A, Tong G, Fang-Newmeyer T, Parker J, Holland EA, Zhang D, Nakanishi N, Chen HS, Wolosker H, Wang Y, Parsons LH, Ambasudhan R, Masliah E, Heinemann SF, Pina-Crespo JC, Lipton SA (2013) Abeta induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A 110:E2518–E2527
Tanti A, Belzung C (2010) Open questions in current models of antidepressant action. Br J Pharmacol 159:1187–1200
Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarapandian MM, Balel C, Wang M, Jia N, Lew F, Chan SL, Chen Y, Lu Y (2010) DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell 140:222–234
Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409
Wan LB, Levitch CF, Perez AM, Brallier JW, Iosifescu DV, Chang LC, Foulkes A, Mathew SJ, Charney DS, Murrough JW (2015) Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry 76:247–252
Xiong Z, Fujita Y, Zhang K, Pu Y, Chang L, Ma M, Chen J, Hashimoto K (2019) Beneficial effects of (R)-ketamine, but not its metabolite (2R,6R)-hydroxynorketamine, in the depression-like phenotype, inflammatory bone markers, and bone mineral density in a chronic social defeat stress model. Behav Brain Res 368:111904
Zanos P, Gould TD (2018) Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 23:801–811
Zhang L, Xu T, Wang S, Yu L, Liu D, Zhan R, Yu SY (2013) NMDA GluN2B receptors involved in the antidepressant effects of curcumin in the forced swim test. Prog Neuro-Psychopharmacol Biol Psychiatry 40:12–17
Zhang W, Sun Q, Jia L, Li M (2019) Ketamine exerts a protective role in a cell-based model of major depressive disorder via the inhibition of apoptosis and inflammation and activation of the Krebs cycle. Bosn J Basic Med Sci
Zhu WL, Wang SJ, Liu MM, Shi HS, Zhang RX, Liu JF, Ding ZB, Lu L (2013) Glycine site N-methyl-D-aspartate receptor antagonist 7-CTKA produces rapid antidepressant-like effects in male rats. J Psychiatry Neurosci 38:306–316
Funding
The work was supported by the National Key Research and Development Program of China (2016YFC1307100), the National Natural Science Foundation of China (81771465, 81930033), the National Key Technologies R&D Program of China (2012BAI01B04); the Sanming Project of Medicine in Shenzhen (SZSM201612006), Youth project of Shanghai Municipal Health and Planning Commission (20154Y0045) and Shanghai Mental Health Center Medical Youth Talents “Flying Plan” (2018-FX-03), and also supported by the Innovative Research Team of High-level Local Universities in Shanghai.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
These authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yao, Y., Ju, P., Liu, H. et al. Ifenprodil rapidly ameliorates depressive-like behaviors, activates mTOR signaling and modulates proinflammatory cytokines in the hippocampus of CUMS rats. Psychopharmacology 237, 1421–1433 (2020). https://doi.org/10.1007/s00213-020-05469-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05469-0