Abstract
Rationale
Changing antipsychotics of patients with chronic schizophrenia involves several risks. Switching to aripiprazole is especially difficult. We investigated switching methods and related factors for successful switching patients with chronic schizophrenia to aripiprazole.
Objectives
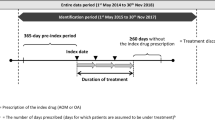
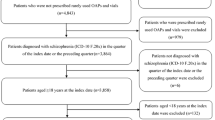
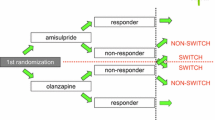
This study was a multi-center historical cohort study and approved by the research ethics committee of Okayama University Hospital and Okayama Psychiatric Medical Center. We compared survival proportions of 178 chronic schizophrenia patients who continued aripiprazole monotherapy for 6 months after non-direct switching (add-on switching (n = 45), cross switching (n = 62)) or direct switching (n = 71). We adjusted possible confounders using a Cox proportional hazards model.
Results
Of patients with chronic schizophrenia, 56.7% (101/178) were switched to aripiprazole monotherapy, and 55.0% (98/178) showed improvement in symptoms as demonstrated by the Clinical Global Impression Severity score. Kaplan-Meier survival curves showed that non-direct switching had a higher survival proportion than direct switching (log-rank test, p = 0.012). Even after adjusting for several variables using a Cox proportional hazards model, add-on switching had a significantly lower hazard at 6 months than direct switching (hazard ratio 0.42, 95% confidence interval 0.21–0.82, P = 0.01). In cases of switching to aripiprazole for psychiatric symptoms, non-direct switching had a lower hazard than direct switching (hazard ratio 0.41, 95% confidence interval 0.21–0.81, P = 0.01) but was not significant for adverse reaction. When aripiprazole was switched from olanzapine, add-on switch showed the lowest hazard ratio for continuation (hazard ratio 0.29, 95% confidence interval 0.07–1.11, P = 0.07).
Conclusions
Flexibility in strategies when switching to aripiprazole may induce a better outcome for patients with chronic schizophrenia.


Similar content being viewed by others
References
Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB (2002) Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 302:381–389
Busner J, Targum SD (2007) The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 4:28–37
Caqueo-Urízar A, Gutiérrez-Maldonado J, Miranda-Castillo C (2009) Quality of life in caregivers of patients with schizophrenia: a literature review. Health Qual Life Outcomes 7:84
Casey DE, Carson WH, Saha AR et al (2003) Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology 166:391–399
Cerovecki A, Musil R, Klimke A, Seemüller F, Haen E, Schennach R, Kühn KU, Volz HP, Riedel M (2013) Withdrawal symptoms and rebound syndromes associated with switching and discontinuing atypical antipsychotics: theoretical background and practical recommendations. CNS Drugs 27:545–572
Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, McGrath J, Whiteford HA (2018) Global epidemiology and burden of schizophrenia: findings from the Global Burden of Disease Study 2016. Schizophr Bull 44:1195–1203
Gründer G, Fellows C, Janouschek H et al (2008) Brain and plasma pharmacokinetics of aripiprazole in patients with schizophrenia: an [18F]fallypride PET study. Am J Psychiatry 165:988–995
Haddad PM, Correll CU (2018) The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol 8:303–318
Hamamura T, Harada T (2007) Unique pharmacological profile of aripiprazole as the phasic component buster. Psychopharmacology 191:741–743
Hor K, Taylor M (2010) Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol 24:81–90
Hwang TJ, Lo WM, Chan HY, Lin CF, Hsieh MH, Liu CC, Liu CM, Hwu HG, Kuo CH, Chen WJ (2015) Fast versus slow strategy of switching patients with schizophrenia to aripiprazole from other antipsychotics. J Clin Psychopharmacol 35:635–644
Inada T, Inagaki A (2015) Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci 69:440–447
Ito C, Kubota Y, Sato M (1999) A prospective survey on drug choice for prescriptions for admitted patients with schizophrenia. Psychiatry Clin Neurosci 53(Suppl):S35–S40
Jordan S, Koprivica V, Chen R, Tottori K, Kikuchi T, Altar CA (2002) The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol 441:137–140
Kegeles LS, Slifstein M, Frankle WG, Xu X, Hackett E, Bae SA, Gonzales R, Kim JH, Alvarez B, Gil R, Laruelle M, Abi-Dargham A (2008) Dose-occupancy study of striatal and extrastriatal dopamine D2 receptors by aripiprazole in schizophrenia with PET and [18F] fallypride. Neuropsychopharmacology 33:3111–3125
Kinon BJ, Chen L, Ascher-Svanum H, Stauffer VL, Kollack-Walker S, Zhou W, Kapur S, Kane JM (2010) Early response to antipsychotic drug therapy as a clinical marker of subsequent response in the treatment of schizophrenia. Neuropsychopharmacology 35:581–590
Kirschbaum KM, Müller MJ, Malevani J, Mobascher A, Burchardt C, Piel M, Hiemke C (2008) Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J Biol Psychiatry 9:212–218
Kishimoto T, Watanabe K, Uchida H et al (2013) Antipsychotic polypharmacy: a Japanese survey of prescribers’ attitudes and rationales. Psychiatry Res 209:406–411
Koue T, Kubo M, Funaki T, Fukuda T, Azuma J, Takaai M, Kayano Y, Hashimoto Y (2007) Nonlinear mixed effects model analysis of the pharmacokinetics of aripiprazole in healthy Japanese males. Biol Pharm Bull 30:2154–2158
Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, Mailman RB (1999) Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology 20:612–627
Leucht S, Arbter D, Engel RR, Kissling W, Davis JM (2009) How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry 14:429–447
Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962
McGrath J, Saha S, Chant D, Welham J (2008) Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 30:67–76
McQuade RD, Stock E, Marcus R et al (2004) A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double-blind study. J Clin Psychiatry 65:47–56
Mueser KT, McGurk SR (2004) Schizophrenia. Lancet 363:2063–2072
Newcomer JW (2007) Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry 68:8–13
Oakley P, Kisely S, Baxter A, Harris M, Desoe J, Dziouba A, Siskind D (2018) Increased mortality among people with schizophrenia and other non-affective psychotic disorders in the community: a systematic review and meta-analysis. J Psychiatr Res 102:245–253
Owen MJ, Sawa A, Mortensen PB (2016) Schizophrenia. Lancet 388:86–97
Pae CU, Serretti A, Chiesa A, Mandelli L, Lee C, Lee C, Kim J, de Ronchi D, Paik IH (2009) Immediate versus gradual suspension of previous treatments during switch to aripiprazole: results of a randomized, open label study. Eur Neuropsychopharmacol 19:562–570
Park JI, Cho DH, Hahn SW, Jeong B, Kim JH, Kim SW, Koo MS, Lee SH, Lee SJ, Lee YH, Park JI, Rho SH, Chung YC (2014) The advantage of using 3-week data to predict response to aripiprazole at week 6 in first-episode psychosis. Int Clin Psychopharmacol 29:77–85
Potkin SG, Saha AR, Kujawa MJ, Carson WH, Ali M, Stock E, Stringfellow J, Ingenito G, Marder SR (2003) Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 60:681–690
Robinson DG, Woerner MG, Delman HM, Kane JM (2005) Pharmacological treatments for first-episode schizophrenia. Schizophr Bull 31:705–722
Ryckmans V, Kahn JP, Modell S, Werner C, McQuade R, Kerselaers W, Lissens J, Sanchez R (2009) Switching to aripiprazole in outpatients with schizophrenia experiencing insufficient efficacy and/or safety/tolerability issues with risperidone: a randomized, multicentre, open-label study. Pharmacopsychiatry 42:114–121
Sakamoto S, Takaki M, Okahisa Y, Mizuki Y, Inagaki M, Ujike H, Mitsuhashi T, Takao S, Ikeda M, Uchitomi Y, Iwata N, Yamada N (2016) Individual risk alleles of susceptibility to schizophrenia are associated with poor clinical and social outcomes. J Hum Genet 61:329–334
Shahid M, Walker GB, Zorn SH, Wong E (2009) Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol 23:65–73
Shiovitz TM, Welke TL, Tigel PD, Anand R, Hartman RD, Sramek JJ, Kurtz NM, Cutler NR (1996) Cholinergic rebound and rapid onset psychosis following abrupt clozapine withdrawal. Schizophr Bull 22:591–595
Takase M, Kanahara N, Oda Y, Kimura H, Watanabe H, Iyo M (2015) Dopamine supersensitivity psychosis and dopamine partial agonist: a retrospective survey of failure of switching to aripiprazole in schizophrenia. J Psychopharmacol 29:383–389
Takeuchi H, Suzuki T, Uchida H, Nakajima S, Nomura K, Kikuchi T, Manki H, Watanabe K, Kashima H (2008) A randomized, open-label comparison of 2 switching strategies to aripiprazole treatment in patients with schizophrenia: add-on, wait, and tapering of previous antipsychotics versus add-on and simultaneous tapering. J Clin Psychopharmacol 28:540–543
Takeuchi H, Kantor N, Uchida H et al (2017) Immediate vs gradual discontinuation in antipsychotic switching: a systematic review and meta-analysis. Schizophr Bull 43:862–871
Yokoi F, Gründer G, Biziere K, Stephane M, Dogan AS, Dannals RF, Ravert H, Suri A, Bramer S, Wong DF (2002) Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C] raclopride. Neuropsychopharmacol 27:248–259
Acknowledgments
The authors would like to thank the Zikei Institute of Psychiatry (Okayama, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the research ethics committee of Okayama University Hospital and Okayama Psychiatric Medical Center.
Conflict of interest
This work was supported in part by an award from the Dopamine Partial Agonist Society (Tokyo, Japan) (Manabu Takaki).
N.Y. has received unrestricted research funding from Daiichi Sankyo, Eisai, Pfizer, Otsuka, Astellas, and Merck Sharp & Dohme, which was deposited into research accounts at Okayama University. N.Y. has received honoraria for his participation as a speaker at educational events from UCB Japan, Tsumura, Pfizer, Dainippon-Sumitomo, Daiichi-Sankyo, Merck Sharp & Dohme, Pfizer, Eisai, Meiji-Seika, and Mochida.
M.T. has received honoraria for his participation as a speaker at educational events sponsored by Otsuka and Dainippon Sumitomo.
S.S. has received unrestricted research funding from Eli Lilly, which was deposited into research accounts at Okayama University Hospital. S.S. has received honoraria for his participation as a speaker at an educational event sponsored by Otsuka.
B.Y. has received honoraria for his participation as a speaker at educational events sponsored by Janssen.
Y.O., S.M., N.M., T.K., Y.Y, Y.O., S.T., Y.K., and T.T. report no additional financial or other relationship relevant to this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Figure 1:
Schematic diagram of this and previous studies. There were five previous studies of switching strategies of aripiprazole. The methods of these are summarized and compared to our method. Our study (upper left), Pae et al. 2009 (upper middle), Casey et al. 2003 (upper right), Takeuchi et al. 2008 (lower left), Hwang et al. 2015 (lower middle), and Ryckmans et al. 2009 (lower right). (PPTX 265 kb)
Rights and permissions
About this article
Cite this article
Obayashi, Y., Mitsui, S., Sakamoto, S. et al. Switching strategies for antipsychotic monotherapy in schizophrenia: a multi-center cohort study of aripiprazole. Psychopharmacology 237, 167–175 (2020). https://doi.org/10.1007/s00213-019-05352-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05352-7