Abstract
Rationale
Metabotropic glutamate receptors and muscarinic M4 receptors have been proposed as novel targets for various brain disorders, including schizophrenia. Both receptors are coupled to Go/i proteins and are expressed in brain circuits that are important in schizophrenia. Therefore, their mutual activation may be an effective treatment and allow minimizing the doses of ligands required for optimal activity.
Objectives
In the present studies, subactive doses of mGlu4 and M4 activators (LSP4-2022 and VU152100, respectively) were administered to investigate the mutual interaction between mGlu4 and M4 receptors in animal models of schizophrenia.
Methods
The behavioral tests used were MK-801-induced hyperactivity, (±)-2.5-dimethoxy-4-iodoamphetamine hydrochloride (DOI)-induced head twitches, the modified forced swim test, and MK-801-induced disruptions of social interactions and novel object recognition. DOI-induced spontaneous excitatory postsynaptic currents (sEPSCs) in brain slices and positron emission tomography (PET) in were used to establish the ability of these compounds to modulate the glutamatergic and dopaminergic systems. Rotarod was used to assess putative adverse effects.
Results
The mutual administration of subactive doses of LSP4-2022 and VU152100 exerted similar antipsychotic-like efficacy in animals as observed for active doses of both compounds, indicating their additive actions. VU152100 inhibited the DOI-induced frequency (but not amplitude) of sEPSCs in the frontal cortex, confirming presynaptic regulation of glutamate release. Both compounds reversed amphetamine-induced decrease in D2 receptor levels in the striatum, as measured with [18F]fallypride. The compounds did not induce any motor impartments when measured in rotarod test.
Conclusions
Based on our results, the simultaneous activation of M4 and mGlu4 receptors is beneficial in reversing MK-801- and amphetamine-induced schizophrenia-related changes in animals.
Similar content being viewed by others
Introduction
Schizophrenia is a brain disorder that affects approximately 1% of the human population. The disease is less common than other psychiatric disorders, such as depression or anxiety (Global Burden of Disease Study 2013), but is considered as one of the most severe mental health disorders. Schizophrenia is estimated to cause 1% of worldwide disability adjusted life years (DALYs) due to long-term unemployment, poverty, and homelessness (Ormel et al. 2008; Rössler et al. 2005). Unfortunately, only approximately 20% of patients with schizophrenia are effectively treated with current medications. The most treatment-resistant symptoms of schizophrenia are negative and cognitive symptoms, which simultaneously have a greater contribution to a poor quality of life and functional disability than the positive symptoms (Velligan et al. 2009; Kane and Mayerhoff 1989). The efficacy of typical neuroleptics towards positive symptoms of schizophrenia is relatively good, although typical antipsychotics induce a variety of adverse effects due to the blockade of D2 receptors in the striatum, including extrapyramidal motor effects (Corripio et al. 2012; Bo et al. 2016).
Antipsychotic drug discovery constitutes the main field of interest of many research groups, and many potential antipsychotic drug targets have been developed. Metabotropic glutamate receptors (mGlu), which were discovered in 1985, constitute one of such targets (Sladeczek et al. 1985; Schoepp et al. 1999; Nicoletti et al. 2015). At least three mGlu receptors subtypes are considered when developing antipsychotic treatments, including mGlu2, mGlu4, and mGlu5 receptors. The activation of these receptors with agonists or positive allosteric modulators (PAMs) induces antipsychotic-like effects in variety of animal models of schizophrenia (Conn et al. 2009b, c; Wierońska et al. 2016; Poels et al. 2014; Muguruza et al. 2016; Ellaithy et al. 2015; Lindsley and Stauffer 2013; Hashimoto et al. 2013).
The muscarinic acetylcholine (ACh) receptor ligands represent another emerging approach in antipsychotic drug discovery. M1 and M4 are the most heavily expressed in the central nervous system (CNS) and represent attractive therapeutic targets for brain disorders, including schizophrenia (Bymaster et al. 2002; Messer 2002; Raedler et al. 2007). Although the expression of M4 receptors was also observed in peripheral tissues (lungs and enteric neurons), the adverse effects of cholinergic agents are thought to be primarily due to activation of peripheral M2 and M3 mAChRs (Bymaster et al. 2003a, b).
A number of selective M4 receptor ligands were synthesized and their efficacy in treating animal models of several CNS disorders, including schizophrenia, was proposed (Byun et al. 2014; Brady et al. 2008; Dencker et al. 2012). Specific modulators of the M4 receptor, VU152100, VU0152099, or LY2033298, reversed the amphetamine-induced hyperlocomotion in rats (Brady et al. 2008; Suratman et al. 2011), were active in self-administration and cocaine-induced hyperactivity, and enhanced associative learning in rodents (Byun et al. 2014; Dencker et al. 2012; Brady et al. 2008; Bubser et al. 2014).
In the present studies, the synergic antipsychotic action of mGlu4 and M4 receptor activation was investigated. The basic assumption of the study was to establish if the administration of subeffective doses of the ligands of those receptors would exert antipsychotic-like activity without inducing adverse effects typical for standard dopamine-based antipsychotics. Such simultaneous action of the combined treatment was reported previously for subeffective doses of mGlu4-5-HT1A receptor ligands (Wierońska et al. 2013, 2015). In addition, studies were undertaken with the combined administration of mGlu4 and GABAB activators, but the efficacy of subeffective doses of the combination of these ligands was not evident in the models of negative and cognitive symptoms of schizophrenia, although the ligands exerted antipsychotic efficacy when active doses of each compound were administered alone (Wierońska et al. 2015; Woźniak et al. 2016).
Behavioral, neurochemical, and brain imaging techniques were used to assess the putative interaction between M4 and mGlu4 receptors. Ligands with known activity profiles such as VU152100 and LSP4-2022 were used. The activity of the compounds was tested in MK-801- and amphetamine-induced hyperactivity tests, DOI-induced head twitches, social interactions, the modified forced swim test, and novel object recognition tests. The activity of VU152100 on DOI-induced spontaneous excitatory postsynaptic currents (sEPSCs) in the brain slices from frontal cortex was examined. Finally, positron emission tomography (PET) imaging was used to establish whether the drugs were able to reverse the amphetamine-induced decrease in D2 receptor levels in the striatum. Rotarod was used to establish if the compounds induce any adverse effects, alone or in the combinations.
Materials and methods
Animals and housing
Male Albino Swiss mice (18–20 g Charles River Laboratory, Germany) were used in behavioral tests and electrophysiology (see details below). Male Wistar rats (250–300 g, Envigo, Inc., Indianapolis, USA) were used in PET imaging and amphetamine-induced hyperactivity. The animals were housed 4 (rats) and 10 (mice) in standard laboratory cages under a 12:12 light–dark cycle in a room with a temperature of 19–21 °C, 50–60% humidity, and had free access to food and water. All compounds were administered in a volume of 10 ml/kg when given to mice and 1 ml/kg when injected into rats. The experimental assessments were performed by an observer who was blinded to the treatment. The procedures were conducted in accordance with the European Communities Council Directive of 22 September 2010 (2010/63/EU) and Polish legislation acts concerning animal experimentation. The experiments were approved by II Local Ethics Committee in Krakow by the Institute of Pharmacology, Polish Academy of Sciences in Krakow (no. 16/2017; 17/2017) and National Institutes of Health Animal Care and Use Committee approved by the Institutional Animal Care and Use Committee in the USA (microPET, M/15/209 and M/15/206).
Drugs
The following drugs were used: LSP4-2022 (mGlu4 receptor agonist, [(3S)-3-Amino-3-carboxy)propyl][(4-(carboxymethoxy)phenyl)hydroxymethyl]phosphinic acid) was synthesized in Francine Acher’s laboratory. The compound is a derivative of its precursor, LSP1-2111, and was profiled as the best currently available orthosteric agonist of mGlu4 receptors (Goudet et al. 2012; Cajina et al. 2013). No activity at muscarinic receptors (M1–M5) was detected in functional studies, which were performed by DiscoverX (Table 1). The compound was dissolved in saline. The administration schedule for LSP4-2022 was based on our previous studies (Woźniak et al. 2016, 2017). VU152100 (3-Amino-N-(4-methoxybenzyl)-4,6-dimethylthieno[2,3-b]pyridine carboxamide, Tocris Bioscience, Bristol, UK) was dissolved in 10% Tween 80. Dosing of the compound was partially based on the results from previous studies (Byun et al. 2014), as well as on our own dose dependence studies. In the behavioral experiments, subthreshold doses for LSP4-2022 and VU0152100 were used in order to examine the antipsychotic action of simultaneous activation of mGlu4 and M4 receptors. For clear information which dose was subtreshold for each compound, please see Table 2. Both compounds were administered i.p, 30 min (VU152100) or 45 min (LSP4-2022) before DOI, MK-801, amphetamine, or appropriate vehicle administration. MK-801 ((5R,10S)-(-)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cylcohepten-5,10-imine maleate) and DOI (4-iodo-2,5-dimethoxy-α-methylbenzeneethanamine hydrochloride) (Tocris Bioscience, Bristol, UK) were dissolved in 0.9% NaCl and injected i.p. Different doses of MK-801 were applied to obtain optimal effects in each test, which is consistent with our previous studies (Wierońska et al. 2012, 2013; Woźniak et al. 2017) and the studies of other research groups (Geyer and Ellenbroek 2003). Amphetamine ((+)-α-methylphenethylamine hemisulfate salt, Sigma-Aldrich) was dissolved in 0.9% saline and administered s.c. Risperidone (3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one, Tocris Bioscience, Bristol, UK) and haloperidol (4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-1-butanone, WZF Polfa S.A.) were dissolved in 0.2% Tween 80 and administered i.p, 30 min before experiments (based on preliminary experiments and our previous studies Sławińska et al. 2013). All animals that were not treated with drugs (control groups) received appropriate vehicles.
MK-801-induced hyperactivity in mice
Locomotor activity was recorded in locomotor activity cages (according to Rorick-Kehn et al. 2007; Wierońska et al. 2012, 2013). The locomotor activity was recorded individually for each animal in OPTO-M3 locomotor activity cages (Columbus Instrument) linked online to a compatible PC. Each cage (13 cm × 23 cm × 15 cm) was surrounded with an array of photocell beams. Interruptions of these photobeams resulted in horizontal activity defined as ambulation counts. The mice were individually placed into actometers for an acclimation period of 30 min. Then, VU152100 (5 mg/kg) and LSP4-2022 (0.1 mg/kg) were administered. MK-801 was i.p. administered at a dose of 0.35 mg/kg and locomotor activity was measured for 60 min immediately after the injection. All groups were compared with the MK-801 control group. The experiment also included a control group that was not treated with MK-801.
Head twitch test
The experiment was performed according to the methods reported by Wierońska et al. (2012, 2013). Each animal was transferred to a 12 (diameter) × 20 cm (height) glass cage lined with sawdust 30 min before the experiment. The head twitches of the mice were induced by an i.p. injection of DOI (2.5 mg/kg). VU152100 was administered at the doses of 0.5, 1, 2, and 10 mg/kg 30 min before the DOI injection. In the combined administration, VU152100 was administered at the dose 1 mg/kg, while LSP4-2022 was administered at the dose of 0.25 mg/kg. The number of head twitches was counted during a 20-min session immediately after DOI administration.
Social interaction test
The social interaction test was performed according to a previously described method (Oh et al. 2013; de Moura Linck et al. 2008; Woźniak et al. 2017). The body weights of the paired mice were matched to within a 10% difference. Both adaptation (2 days, 10 min of free exploration) and the subsequent test were conducted in black plastic boxes (50 × 30 × 35 cm) illuminated with the light intensity of 335 lx. The social interactions between two mice were determined based on the total time spent participating in social behaviors, such as sniffing, genital investigation, chasing, and fighting each other, during a 10-min test. Each dose of VU152100 (0.5, 2, and 5 mg/kg) was co-administered with subtreshold (0.1 and 0.5 mg/kg) or active (1 mg/kg) dose of LSP4-2022. The doses of LSP4-2022 were chosen according to our previous studies (Woźniak et al. 2017). MK-801 (0.3 mg/kg) was administered 30 min before the test. Control experiments with animals that did not receive MK-801 were conducted to determine whether the drugs had any influence on social behavior when administered alone.
Modified forced swim test
The modified forced swim test was performed according to the method introduced by Noda (Noda et al. 1995, 1997), Wierońska et al. (2015), and Woźniak et al. (2016, 2017). The swim tests were performed in a glass cylinder (height, 20 cm; internal diameter, 15 cm) containing 11 cm of water maintained at 23–26 °C. After the acclimation period, the animals underwent the first swim test, where the immobility time was measured during a 3-min period (T1). On the next day, chronic (13 days) MK-801 administration (0.4 mg/kg, i.p.) was started. After a 1-day break, on the 15th day of experiment, the second swim session was performed and the immobility time during 3-min test was measured again (T2). The T2 − T1 difference was reported as the result of the experiment. Drugs were administered acutely before the T2 session. VU152100 was administered at the doses of 0.5, 1, and 2 mg/kg (30 min before the test), and then the subthreshold dose of the compound (0.1 mg/kg) was co-administered with subthreshold dose of LSP4-2022 (0.1 mg/kg, 45 min before the test).
Novel object recognition
The method was performed as described by Nilsson et al. (2007) and Woźniak et al. (2017). The animals were trained and tested in a black plastic rectangular open field (50 × 30 × 35 cm). The open field was placed in a dark room and was illuminated with only the light intensity of 335 lx. After 2 days of adaptation (10 min of free exploration), the animals were placed in the apparatus on the experimental day and allowed to explore two identical objects (a red, glass cylinder, 6.5 cm in diameter, 4.5 cm high) for 10 min. For the retention trial (T2) that was conducted 1 h later, one of the objects presented in T1 was replaced with a novel object (a transparent glass elongated sphere-like object with an orange cap). The duration of exploration of each object (i.e., sitting in close proximity to the objects or sniffing or touching them) during 5 min was video-recorded and measured separately by a trained observer. The results were calculated as recognition index, defined as (Tnovel − Tfamilial / Tfamilial + Tnovel) × 100. All drugs were administered before the training (T1) session. MK-801 (0.3 mg/kg) was administered 30 min before the session. Each dose of VU152100 (0.25, 0.5, and 1 mg/kg) was co-administered with subtreshold (0.1 and 0.5 mg/kg) or active (1 mg/kg) dose of LSP4-2022. The doses of LSP4-2022 were chosen according to our previous studies (Woźniak et al. 2017). Control experiments with animals that did not receive MK-801 were conducted to determine whether the drugs had any influence on social behavior when administered alone.
DOI-induced sEPSCs
Albino Swiss mice were decapitated; their frontal cortices were dissected and cut into slices (420 μm thick) in the frontal plane using a vibrating microtome. Slices were submerged in artificial cerebrospinal fluid (ACSF) consisting of (in mM) 126 NaCl, 4 KCl, 2.5 CaCl2, 1.3 MgSO4, 1.25 KH2PO4, 26 NaHCO3, and 10 glucose, bubbled with 95% O2/5% CO2, pH = 7.4. A single slice was transferred to the recording chamber (volume 1 ml) and superfused with warmed (32 °C) ACSF at 2 ml/min. Individual neurons were visualized using an upright microscope (Zeiss Axioskop 2FS) equipped with a long-range water immersion objective (×40) and an infrared camera. Recording micropipettes were pulled on a Flaming-Brown puller (P-87; Sutter Instruments, Novato, CA, USA) and had a resistance of 6–8 MΩ. Microelectrodes were filled with (in mM) 130 K-gluconate, 5 KCl, 0.3 CaCl2, 2 MgCl2, 1 EGTA, 10 HEPES, 5 Na2-ATP, 0.4 and Na-GTP, with osmolarity of 290 mOsm and pH = 7.2. Whole-cell recordings were obtained from layer V pyramidal cells in the cortex. After confirming the electrophysiological characteristics of the neurons in current clamp mode, cells were voltage-clamped at − 76 mV and sEPSCs were recorded. Signals were acquired using the SEC 05 L amplifier (NPI, Germany) and digitized using the Digidata 1322 interface (Molecular Devices, Sunnyvale, CA, USA). Drugs stored as concentrated stocks were diluted in ACSF just before the experiment and applied to the superfusate. After achieving a stable control recording for at least 15 min, DOI (10 μM) was applied for 15 min and sEPSCs were recorded (8 min). Next, DOI was applied concurrently with VU152100, LSP4-2022, and VU152100/LSP4-2022 for 15 min and sEPSCs were again recorded. The measured parameters were the frequency and amplitude of sEPSCs. The data were analyzed off-line using the Mini Analysis program (Synaptosoft Inc., ver. 6.0.3).
Amphetamine-induced hyperactivity in rats
Rats were habituated to the locomotor activity cages for 30 min. The locomotor activity was recorded individually for each animal in Opto-Varimex cages (Columbus Instruments, Columbus, OH, USA) connected to a compatible IBM-PC. Each chamber (43 cm × 43 cm × 21 cm) was made of transparent acrylic plastic (all six sides), equipped with a 220 lx house light, and was placed in a light- and soundproof wooden cubicle. The corner brackets were made of stainless steel. Each cage was surrounded by a 15 × 15 array of photocell beams located 3 cm from the floor surface. Interruptions of these photobeams resulted in horizontal activity defined as ambulation counts. The rats were injected with LSP4-2022 (0.1 and 2 mg/kg), VU152100 (2.5, 5, and 15 mg/kg), and with combined treatment of VU152100 (5 mg/kg) and LSP4-2022 (0.1 mg/kg). Amphetamine was administered s.c. at a 1-mg/kg dose and the locomotor activity was measured for 60 min immediately after the injection.
MicroPET imaging
Rats were imaged according to the procedures outlined in a previous work (Tantawy et al. 2009, 2011). Briefly, rats were anesthetized with < 2% isoflurane and injected with ~ 13 MBq/0.2 ml [18F]fallypride, followed by a 0.1-ml of saline via a tail vein catheter. Rats were under anesthesia for less than 10 min. Rats were then returned to their cages and fed ad libitum. Rats returned to full activity within 10–20 min after isoflurane had been removed. Fifty minutes later, rats were anesthetized with < 2% isoflurane and positioned in an Inveon microPET/CT (Siemens, Knoxville TN). A CT scan was initiated with an x-ray beam intensity of 25 mAs and an x-ray peak voltage of 80 kVp, followed by a 60-min dynamic PET scan acquisition. The PET scans always started at 60 min after radiotracer administration. The 60-min dynamic acquisition was divided into six frames of a 600-s duration each. All datasets were reconstructed using the OSEM-2D algorithm into 128 × 128 × 95 slices with a voxel size of 0.095 × 0.095 × 0.08 cm3, after correcting for scatter and attenuation. The resulting images were manually co-registered to an MRI brain template (Rubins et al. 2003; Schweinhardt et al. 2003) using the medical imaging analysis tool AMIDE software (Loening and Gambhir 2003). Anatomical volumetric regions-of-interest (ROIs) were drawn around the left striatum, right striatum, and cerebellum. The radiotracer concentrations within the ROIs were used to estimate the modified distribution volume ratio (DVR′) (Tantawy et al. 2009), where the cerebellum, which expresses few or no D2 receptors, was used as the reference tissue. Percent occupancy was calculated as: percent occupancy = ((DVR′Tvehicle − DVR′Ttreatment) / DVR′Tvehicle) × 100.
Rats were injected with amphetamine (1 mg/kg, s.c.) 15 min prior to the administration of [18F]fallypride. The investigated compounds, LSP4-2022 and VU152100, were administered 45 and 30 min, respectively, before amphetamine administration. Four groups of rats were tested: AMPH+LSP4-2022 (2 mg/kg), AMPH+LSP4-2022 (0.1 mg/kg), AMPH+VU152100 (15 mg/kg), and AMPH+VU152100 (5 mg/kg).
Rotarod test
The rotarod test was performed as described by Vogel et al. (2008) with small modifications. The animals were trained for three consecutive days at the speed of 18 rpm, one session per day for 3 min. If a mouse fell during the habituation period, it was placed back on the apparatus. On the following day, the test trial was performed. After the mice were placed on the apparatus (Mouse Rotarod NG, UGO BASILE S.R.L.) moving at the speed of 12 rpm, the accelerating mode was started (maximum speed 24 rpm). The latency to fall was measured during 3-min test session. Mice were injected with VU152100 (0.5; 5 mg/kg), LSP4-2022 (0.1; 2 and 5 mg/kg), risperidone (0.1; 0.5 mg/kg), or haloperidol (0.2; 1 mg/kg). Then, different combinations of subtreshold doses of VU152100 with LSP4-2022 were administered, as well as subtreshold dose of VU152100 (0.5 mg/kg) and LSP4-2022 (0.1 mg/kg) were co-administered with two doses of haloperidol or risperidone. Mice were administered with the investigated compounds 30 min before the test, except LSP4-2022, which was administered 45 min before the test.
Statistical analysis
The data are presented as means ± SEM. Statistical analyses of the data were performed using the Statistica 10 package (StatSoft Inc., OK, USA). One-way ANOVA followed by the Newman-Keuls post hoc analysis was used in dose dependence studies, and two-way ANOVA followed by the Newman-Keuls post hoc comparison test was used for the interaction studies. Student’s T test was used to determine the significance of the results obtained in electrophysiological recordings. P values < 0.05 were considered statistically significant.
Results
Locomotor activity studies
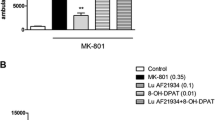
At a dose of 0.35 mg/kg, MK-801 induced a typical increase in locomotor activity (P < 0.001). The administration of subeffective doses of VU152100 (5 mg/kg) and LSP4-2022 (0.1 mg/kg) did not influence MK-801-induced hyperlocomotion. The co-administration of both compounds at the doses indicated above resulted in a statistically significant reversal of MK-801-induced hyperactivity (Fig. 1). The administration of the combination without MK-801 did not have any influence on locomotor activity (Table 3).
Effects of VU152100 and LSP4-2022 on MK-801-induced hyperactivity in mice that had been habituated to locomotor activity cages. LSP4 and VU152100 were administered 45 and 30 min, respectively, before MK-801 administration. Locomotor activity was measured for 60 min immediately after the MK-801 injection. Doses in milligrams per kilogram are indicated in parentheses. Data are presented as means ± SEM. Two-way ANOVA revealed a statistically significant interaction [F(1.35) = 4.69; P < 0.03]. #P < 0.001 compared with the control group. *P < 0.05 compared with the MK-801-treated group. Number of animals in each group n = 10
DOI-induced head twitches
At the doses of 2 and 10 mg/kg, VU152100 induced a significant reduction of DOI-induced head twitches, while it was ineffective at the doses of 0.5 and 1 mg/kg (Fig. 2a). The co-administration of subthreshold doses of both compounds (VU152100 1 mg/kg and LSP4-2022 0.25 mg/kg) partially antagonized DOI-induced effect, but the effect was not statistically significant (Fig. 2b).
Effects of VU152100 (a) and the combined administration of VU152100 and LSP4-2022 (b, c) on DOI-induced head twitches. LSP4 and VU152100 were administered 45 and 30 min, respectively, before DOI administration. The number of head twitches was measured for 20 min immediately after DOI administration. Doses in milligrams per kilogram are indicated in parentheses. Data are presented as means ± SEM. One-way ANOVA [F(4.31) = 7.05; P < 0.0004], **P < 0.01 and ***P < 0.005 compared with the control group. Number of animals in each group n = 7. The effect of LSP4-2022/VU152100 (c) did not reach statistical significance [F(1.29) = 1.07, P = 0.3]
Social interaction
At a dose of 0.3 mg/kg, MK-801 induced a disruption of social behaviors, as observed in the decrease in the duration of social contacts and in the number of episodes. At a dose of 5 mg/kg, VU152100 clearly reversed the MK-801-induced effects on both the time of interaction and the number of episodes. The administration of 0.5 and 2 mg/kg doses of VU152100 was ineffective (Fig. 3a).
Effects of VU152100 (VU) and LSP4-2022 (LSP4) on MK-801-induced social interaction deficits. The time spent in social interactions was measured. a Effects of VU152100 administration and (b) effects of the combined administration of all three doses of VU152100 with LSP4 at subthreshold (0.1 and 0.5) and active (1) doses are shown. LSP4 and VU152100 were administered 45 and 30 min, respectively, before MK-801 administration. Doses in milligrams per kilogram are indicated in parentheses. Data are presented as means ± SEM. One-way ANOVA [F(4.27) = 12.18; P < 0.01 and F(4.27) = 11.39; P < 0.01] (a) and two-way ANOVA analysis [&F(1.27) = 5.08; P < 0.03 (versus LSP4 0.1 mg/kg and VU 0.5 mg/kg) and && F(1.27) = 27,05; P < 0.00005 (versus LSP4 0.5 mg/kg and VU 0.5 mg/kg)] (b). #P < 0.01 compared with the control group, ***P < 0.0001 compared with the MK-801-treated group, &P < 0.01 compared to LSP4 (0.1 mg/kg) and VU (0.5 mg/kg) treated groups, &&P < 0.0001 compared to LSP4 (0.5 mg/kg) and VU (0.5 mg/kg) treated groups. Number of animals in group varied n = 8–10
The co-administration of subthreshold doses of both VU152100 (2 mg/kg) and LSP4-2022 (0.1 or 0.5 mg/kg) totally reversed the effect of MK-801 in a way comparable to the effect of the most active dose of VU152100 alone (Fig. 3b). The co-administration of VU152100 at the higher dose 2 mg/kg with subthreshold doses of LSP4-2022 (0.1 and 0.5 mg/kg) also reversed the action of MK-801 to the level achieved by the administration of the most active dose of VU152100 or LSP4-2022 alone. The action of the most active dose of VU152100 (5 mg/kg) was not enhanced when co-administered with LSP4-2022 at all three doses (Fig. 3b).
Neither VU152100 nor the combination of subtreshold doses of VU152100 with LSP4-2022 changed the behavior of animals when administered in the absence of MK-801 (Table 3).
Modified forced swim test
Chronic administration of MK-801 increased the immobility time in T2 session. The results are shown as a difference in the immobility time between T2 and T1 sessions (P < 0.01). VU152100 reversed this MK-801-induced effect at doses of 0.5, 1, and 2 mg/kg (Fig. 4a). The co-administration of the ineffective dose of the compound (0.1 mg/kg) together with a subthreshold dose of LSP4-2022 (0.1 mg/kg) displayed the same effect as the active doses of VU152100 (Fig. 4b). The spontaneous locomotor activity was not changed after the MK-801 administration or after the VU152100 or VU152100+LSP4-2022 administration (Table 3).
Effects of VU152100 (a) and the combined administration of VU152100 with LSP4 (b) on the immobility time in the modified forced swim test after chronic administration (13 days) of MK-801. Doses in milligrams per kilogram are indicated in parentheses. Data are presented as means ± SEM. #One-way ANOVA [F(3.36) = 15.72; P < 0.001] (a) and two-way ANOVA of the effects [F(1.36) = 4.99; P < 0.05], #P < 0.01 compared with the control group, **P < 0.02 and ***P < 0.001 compared with the MK-801-treated group. Number of animals in each group n = 10
Novel object recognition test
MK-801 induced a disruption in the novel object recognition behavior, as measured by the recognition index (P < 0.001). VU152100 reversed this MK-801-induced effect at doses of 0.5 and 1 mg/kg. The administration of a 0.25-mg/kg dose of the compound was ineffective (Fig. 5a).
Effects of VU152100 (a), the combined administration of VU152100 with LSP4 (b and c) and the administration of VU152100 to mGlu4 KO mice (d) on MK-801-induced deficits in the NOR test. LSP4 and VU152100 were administered 45 and 30 min, respectively, before MK-801 administration. Doses in milligrams per kilogram are indicated in parentheses. Data are presented as means ± SEM. One-way ANOVA [F(3.26) = 9.62; P < 0.01] (a) and two-way ANOVA of main effects [F(1.29) = 5.17; P < 0.0002] (b). #P < 0.001 compared with the control group, *P < 0.05, ***P < 0.001 compared with the MK-801-treated group, &P < 0.05 compared with LSP4 (1 mg/kg) and VU (0.25 mg/kg) treated groups. Number of animals in each group n = 8–10
The co-administration of subthreshold doses of VU152100 (0.25 mg/kg) and LSP4-2022 (1 mg/kg) induced a clear antipsychotic-like effect, similar to the highest effective doses of VU152100 (Fig. 5b). The co-administration of 0.25 mg/kg VU152100 with active doses of LSP4-2022 or the co-administration of active doses of VU152100 (0.5 and 1 mg/kg) with three doses of LSP4-2022 (1, 2, and 4 mg/kg) reversed the action of MK-801 to the level achieved by the administration of the most active dose of VU152100 or LSP4-2022 alone (Fig. 5b). No enhancement of the activity of active doses of each compound was observed.
Neither VU152100 nor the combination of subeffective doses of VU152100 with LSP4-2022 changed the behavior of animals when administered without MK-801 (Table 3).
DOI-induced spontaneous sEPSCs
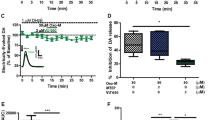
Voltage-clamp recordings were obtained from layer V cortical cells in the presence of picrotoxin (30 μM), which blocks GABAA receptor-mediated currents, to investigate the effects of DOI on sEPSCs. All recorded cells (n = 69) had electrophysiological characteristics of regular spiking pyramidal neurons (tested in current clamp; McCormick et al. 1985). Their mean resting membrane potential (RMP) was − 74 ± 5 mV and the mean input resistance (Rin) was 252 ± 27 MΩ. The mean basal frequency of spontaneous synaptic activity ranged from 2.9 to 7.5 Hz (4.9 ± 0.3 Hz) and its mean amplitude was 9.77 ± 0.3 pA. sEPSCs were blocked by CNQX (5 μM), indicating that they were mediated by AMPA/kainate glutamate receptors (data not shown). The application of DOI (10 μM) systematically increased the mean sEPSC frequency, with an effect ranging from 127 ± 3.151 to 133 ± 3.312% of the control.
Based on the measurements obtained from a separate group of five neurons, the effect of DOI on sEPSCs was not desensitized after 40 min of continuous application of DOI (Figs. 6a and 7a–c).
Effects of VU152100 on DOI-induced spontaneous EPSCs. a Examples of recordings from a representative neuron: (1) control activity, (2) recording obtained after a 10-min incubation with DOI, and (3) recording obtained after a 10-min incubation with VU152100 in the presence of DOI. b VU152100 (50 μM) suppressed the effect of DOI on the mean frequency of the sEPSCs. Data are presented as means ± SEM. Statistical analysis: t = 6.015; df = 9; *P < 0.0001 compared with the DOI-incubated group (concentration of VU152100 = 5 mM; N = 3, n = 11, 10 mM; N = 3, n = 9, 50 mM; N = 3, n = 10). N animal number, n cell number
Effects of LSP4-2022 (LSP) and/or VU152100 (VU) administration on DOI-induced increase in sEPSC frequency. While 1 μM LSP (a) or 5 μM VU (b) applied alone does not change the effect of DOI, joint application of 1 μM LSP and 5 μM VU (c) results in a weakening of DOI-induced increase in sEPSC frequency. d Mean ± SEM sEPSC frequency and amplitude in all experimental groups. Statistical analysis: N = 3, n = 7; t = 6.16; df = 6; *P < 0.001, compared with a respective DOI-incubated cells. Labels in a–c: (1) control activity, (2) recording obtained after a 10-min incubation with DOI, and (3) recording obtained after a 10-min incubation with LSP and/or VU in the presence of DOI. Scale bars in a refer also to b and c. (LSP; N = 3, n = 7, VU152100; N = 3, n = 11, LSP+VU152100; N = 3, n = 7). N animal number, n cell number
Three concentrations of VU152100 (5, 10m and 50 μM) were applied concurrently with DOI. The administration of 50 μM VU152100 reversibly suppressed the DOI-induced increase in the frequency but did not affect the mean amplitude of sEPSCs (n = 10; t = 6.015; df = 9; P < 0.0001) (Fig. 6b). LSP4-2022 when given in not effective dose (1 μM) together with non-effective dose of VU152100 (5 μM) significantly reversed the effect of DOI (n = 7; t = 6.16; df = 6; P < 0.001) (Fig. 7d).
Amphetamine-induced hyperactivity
The administration of a 1-mg/kg dose of amphetamine induced a robust increase in locomotor activity. VU152100 was injected at doses of 2.5, 5, and 15 mg/kg and reversed the action of amphetamine at the highest doses (Fig. 8a), whereas LSP4-2022 was effective at the dose of 2 mg/kg (Fig. 8b).
Effects of VU152100 (a), LSP4 (b), and the combined administration of VU152100 with LSP4 (c) on amphetamine-induced hyperactivity in rats that had been habituated to locomotor activity cages. LSP4 and VU152100 were administered 45 and 30 min, respectively, before amphetamine (AMPH) administration. Locomotor activity was measured for 60 min immediately after AMPH injection. Doses in milligrams per kilogram are indicated in parentheses. Data are presented as means ± SEM. One-way ANOVA [F(3.30) = 54.65; P < 0.0001] (a) and [F(2.25) = 5.74; P < 0.01] (b). Number of animals in groups n = 8–10. Two-way ANOVA of the effects of the interaction [F(1.31) = 6.1; P < 0.02]. #P < 0.001 compared with the control group, ***P < 0.0001, **P < 0.01, and *P < 0.05 compared with the AMPH-treated group. Number of animals in groups n = 8–10
The co-administration of both compounds at subthreshold doses (LSP4-2022 0.1 mg/kg and VU152100 5 mg/kg) significantly reduced amphetamine-induced hyperactivity (Fig. 8c).
Impact of LSP4-2022 and VU152100 on D2 receptor occupancy by [18F]fallypride measured using microPET
The DVR′ measured in control rats was 14.56 ± 0.59. Amphetamine administration (1 mg/kg) induced a significant reduction in the DVR′ estimates of up to 23%, which was 11.14 ± 0.84 of control. LSP4-2022 and VU152100 reversed the amphetamine-induced effects at the highest doses (2 and 15 mg/kg, respectively) (Fig. 9a). These compounds did not have any effect on DVR′ estimates when administered alone (Fig. 9b). Representative images and Logan plots are shown in Fig. 10.
Distribution volume ratio (DVR′) estimates of rats injected with [18F]fallypride and imaged in the microPET for 60 min. Results are presented as means ± SEM. #P < 0.005 compared with the controls and *P < 0.05 compared with the amphetamine-treated group. Data are presented as mean standard uptake values ± SEM. Number of animals in groups n = 6 except LSP4 (0.1) and VU (5) where n = 3
Representative positron emission tomography images of [18F]fallypride binding in vehicle- (a) and amphetamine-treated (b) rat brains. Representative Logan plots for vehicle- (c) and amphetamine-treated rats (d). The statistical analysis revealed F(2.8) = 3.79; P < 0.05 for LSP4-2022 and F(2.8) = 5.44; P < 0.05 for VU152100
Motor coordination
In the rotarod test, neither of tested drugs at any dose significantly influenced motor coordination of mice (Fig. 11a). Standard neuroleptics, risperidone (0.1 and 0.5 mg/kg) and haloperidol at the higher dose 1 mg/kg disturbed motor coordination of animals (Fig. 11b). The simultaneous administration of LSP4-2022 and VU152100 had no effect on the behavior of animals as well (Fig. 11c). However, the co-administration of both drugs in subeffective doses with subeffective dose of haloperidol (0.2) disturbed motor coordination in a statistically significant manner. The co-administration of subeffective dose of LSP4-2022 (0.1) with subeffective dose of haloperidol (0.2) also disturbed motor coordination, but such an effect was not observed when LSP4-2022 (0.1) was co-administered with subeffective dose of risperidone (0.1) (Fig. 11d).
Effects of risperidone and haloperidol (a), LSP4-2022 (LSP4), and VU152100 (VU) (b) and the combination of subeffective doses of drugs together (c) or in the combination with standard neuroleptics (d) on rotarod performance in mice. Doses in milligrams per kilogram are indicated in parentheses. Data are presented as means ± SEM. One-way ANOVA revealed statistically significant effect of both doses of risperidone [F(2.20) = 25.88, P < 0.01] and of haloperidol [F(2.20) = 4.7, P < 0.05]. Number of animals in risperidone groups n = 5–6 and in controls and haloperidol 8–10. The effect of combined treatment of LSP4-2022 with risperidone or haloperidol also disturbed motor coordination [F(2.23) = 9.37, *P < 0.05 and **P < 0.01], similarly as the combination of both neuroleptics with VU152100 [F(2.24) = 19.16, P < 0.01]. Number of animals in groups n = 8–10
Discussion
In the present paper, the synergic/mutual interaction between muscarinic M4 and metabotropic glutamatergic mGlu4 receptors was examined in animal models of schizophrenia.
This is a follow-up study of our previous research on antipsychotic-like activity of mGlu4 receptor orthosteric agonists and PAMs (Wierońska et al. 2010, Wierońska et al. 2012, 2013, Woźniak et al. 2016, 2017). In this set of studies, mGlu4 agonist and M4 PAM were investigated. The subthreshold doses of the mGlu4 agonist LSP4-2022 and the M4 positive allosteric modulator VU152100 were administered simultaneously to investigate the putative mutual interaction between mGlu4 and M4 receptors. This combination exhibited efficacy similar to that observed for the administration of active doses of each compound alone in reversing hyperactivity in mice and rats, in a social interaction test, modified forced swim test, and in novel object recognition test. The effect observed in DOI-induced head twitches was clear but did not reach statistical significance. In social interaction and novel object recognition tests, we did more extensive research and each dose of VU152100 was co-administered with subtreshold and active dose of LSP4-2022, which were selected on the basics of our previous studies (Woźniak et al. 2017). The results indicate that only the simultaneous administration of subthreshold doses of both compounds reverses MK-801-induced deficits, and no enhancement of the activity of active doses was observed when they were co-administered with either active or subtreshold dose of the other compound.
There are some reports on the activity of each compound published so far. LSP4-2022, one of the best orthosteric agonists of the mGlu4 receptor, was previously used in our laboratory in both mice and rats in variety of behavioral and neurochemical studies (Woźniak et al. 2016, 2017). VU152100 was introduced in 2008 (Brady et al. 2008) and is one of the two commercially available selective M4 positive allosteric modulators. All behavioral studies that have been published with this compound have predominantly been performed in rats and were performed in dopaminomimetic-based animal models (Brady et al. 2008; Byun et al. 2014; Dencker et al. 2012; Galloway et al. 2014). No published studies have examined the activity of the compound in MK-801-based animal models of schizophrenia, although the administration of an NMDA antagonist better resembles schizophrenia arousal than dopaminomimetics (Javitt 2004; Conn et al. 2009c; Moghaddam and Jackson 2003, 2012). Therefore, in the present research, dose dependence studies showing activity of the compound in MK-801-based models of negative and cognitive symptoms of schizophrenia were carried out for the first time. In our experiments, lower doses of VU152100 were active compared to the results of these earlier reports.
In the second part of the studies, selected actions of VU152100 and LSP4-2022 on glutamatergic and dopaminergic neurotransmission were investigated, using patch-clamp recordings and PET imaging studies.
Earlier, it was shown that LSP4-2022 reversed DOI-induced increases in both frequency and the amplitude of spontaneous EPSCs, confirming its ability to restore DOI-induced increases in glutamatergic system activity (Woźniak et al. 2017). Here we show that VU152100 attenuates the increase of sEPSC frequency triggered by DOI (via activation of postsynaptic 5-HT2A/2C receptors) in layer V pyramidal neurons in cortical slices. Similar effect was observed when both compounds were applied simultaneously at the subthreshold doses. The result is in line with behavioral observation, in which simultaneous action of both ligands was also observed, although in not statistical manner. The attenuation of glutamate-induced sEPSC frequency indicates that the compounds exert their action via presynaptic mechanism (van der Kloot 1991). Previously, in the paper of Pancani et al., it was shown that VU152100 potentiated CCh-induced depression of EPSCs via an increase in paired pulse ratio, thereby indicating that M4-mediated depression of EPSCs in medium spiny neurons (more than 95% of all striatal neuronal population (Kreitzer 2009)) is probably due to decrease in presynaptic glutamate release (Pancani et al. 2014). Comparing to this paper, in our studies, much higher dose of the compound was needed to inhibit DOI-induced sEPSCs in the cortical slices.
Considering the mechanism by which VU152100 exerts its action on DOI-induced head twitches, it should be mentioned that cholinergic interneurons exert powerful modulation of circuit activity within the brain, and M4 receptors expressed on their terminals play essential role in the regulation of acetylcholine release. This released acetylcholine can reciprocally activate dopaminergic neuronal activity via nicotinic receptors, and both agonists and antagonists of nicotinic receptors reverse DOI-induced head twitches (Tizabi et al. 2001). Therefore, the precise mechanism by which cholinergic system is involved in the inhibition of DOI-induced head twitches, except corticostriatal transmission, is yet to be established.
Subsequently, the effects of VU152100 and LSP4-2022 were investigated on D2 receptors in the striatum with PET imaging studies (Tantawy et al. 2009, 2011). Amphetamine administration increased dopamine release and subsequently increased the occupancy of D2 receptors in the striatum, thereby reducing the number of unoccupied D2 receptors (Tantawy et al. 2009, 2011). Active doses of both investigated compounds reversed this amphetamine-induced effect. The fact that both drugs with nondopaminergic mechanism of action are able to restore amphetamine-induced changes in striatum seems to be of importance, as for years, the hyperactivity of the dopaminergic system in the striatum has been regarded as the primary factor triggering the onset of positive symptoms of schizophrenia (Haracz 1982; Heinz and Schlagenhauf 2010). Therefore, the reversal of dopaminergic dysfunction in this structure is crucial for antipsychotic efficacy. However, chronic blockade of D2 receptors in the striatum contributes to the development of adverse effects observed after standard neuroleptics. Thus, compounds that inhibit glutamatergic and dopaminergic neurotransmission without direct blockade of D2 receptors are desired as novel antipsychotics (Fervaha et al. 2015, 2016). It seems that both M4 and mGlu4 PAMs fulfill these criteria and may not induce adverse effects typical for standard neuroleptics. Earlier it was shown that both compounds reversed haloperidol-induced catalepsy and/or did not induce catalepsy by themselves (Goudet et al. 2012; Byun et al. 2014). Here we used rotarod test to establish if the compounds influence motor coordination in animals. Neither LSP4-2022 nor VU152100 impaired balance and motor coordination when administered at the doses higher than those that were effective in behavioral studies. Also the combinations of the compounds in subtreshold doses did not influence the rotarod performance. Standard neuroleptics (haloperidol, risperidone) impaired rotarod performance and the administration of low/subtreshold doses of those neuroleptics with subtreshold doses of VU0152100 or LSP4-2022 also affected motor coordination in mice. It should be mentioned that performance on the rotarod allows assessing one aspect of antipsychotic-induced adverse effects. However, the most relevant measures on long-term treatment with neuroleptics are tardive dyskinesia (involuntary, repetitive body movements, such as grimacing, sticking out the tongue, or smacking of the lips) which results primarily from neuroleptic-induced dopamine supersensitivity in the nigrostriatal pathway, with the D2 dopamine receptor being most affected (Carbon et al. 2017). Therefore, it seems that the optimal pharmacological interventions in schizophrenic patients should omit direct blockade of dopaminergic receptors in the striatum. Simultaneous administration of M4/mGlu4 receptors can be proposed as one of the directions. Except the activity of the ligands on glutamatergic and/or dopaminergic system presented here, it was also shown that their administration reduced amphetamine or MK-801-induced dopamine release in the striatum or prefrontal cortex (Byun et al. 2014; Woźniak et al. 2017).
Neither of the drugs that are approved and currently used in the clinic stimulates mGlu4 and/or M4 receptors. We propose to combine the two ligands and minimize the doses used to reduce the risk of overdosing and omitting putative adverse effects that could develop. Both receptors investigated here are coupled to Go/i signaling and are expressed in the brain circuits involved in schizophrenia, including the striatum, cortex, and hippocampus (Hersch et al. 1994; Levey et al. 1991, 1995). The M4 receptor regulates the activity of dopaminergic and/or acetylcholinergic neurons in the striatum and nucleus accumbens (Ince et al. 1997; Jeon et al. 2010; Dencker et al. 2012; Nadal et al. 2016; Pancani et al. 2014; Bell et al. 2013; Kuroiwa et al. 2012). Similar action may exert mGlu4 receptors (Pancani et al. 2014). Therefore, the ligands may complement each other’s action and putatively be active in subjects with lower expression of mGlu4 and /or M4 receptors or in subjects with partially impaired function of those receptors.
Abbreviations
- DALYs:
-
Disability adjusted life years
- CNS:
-
Central nervous system
- mGlu:
-
Metabotropic glutamate
- PAM:
-
Positive allosteric modulator
- Ach:
-
Acetylcholine
- M:
-
Muscarinic
- PET:
-
Positron emission tomography
- KO:
-
Knockout
- NOR:
-
Novel object recognition
- DVR:
-
Distribution volume ratio
- ROI:
-
Region of interest
References
Bell LA, Bell KA, McQuiston AR (2013) Synaptic muscarinic response types in hippocampal CA1 interneurons depend on different levels of presynaptic activity and different muscarinic receptor subtypes. Neuropharmacol 73:160–173
Bo QJ, Li XB, Wang ZM, Li AN, Ma X, Wang CY (2016) Extrapyramidal symptoms during risperidone maintenance treatment in schizophrenia: a prospective, multicenter study. J Clin Psychopharmacol 36:125–129
Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, Breininger ML, Gentry PR, Yin H, Jadhav SB, Shirey JK, Conn PJ, Lindsley CW (2008) Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-induced hyperlocomotor activity in rats. J Pharmacol Exp Ther 327:941–953
Bubser M, Bridges TM, Dencker D, Gould RW, Grannan M, Noetzel MJ, Lamsal A, Niswender CM, Daniels JS, Poslusney MS, Melancon BJ, Tarr JC, Byers FW, Wess J, Duggan ME, Dunlop J, Wood MW, Brandon NJ, Wood MR, Lindsley CW, Conn PJ, Jones CK (2014) Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem Neurosci 5:920–942
Bymaster FP, Felder C, Ahmed S, McKinzie D (2002) Muscarinic receptors as a target for drugs treating schizophrenia. Curr Drug Targets 1:163–181
Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC (2003a) Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci 17:1403–1410
Bymaster FP, McKinzie DL, Felder CC, Wess J (2003b) Use of M1-M5 muscarinic receptor knockout mice as novel tools to delineate the physiological roles of the muscarinic cholinergic system. Neurochem Res 28:437–442
Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J, Gowrishankar R, Kelm ND, Damon S, Bridges TM, Melancon BJ, Tarr JC, Brogan JT, Avison MJ, Deutch AY, Wess J, Wood MR, Lindsley CW, Gore JC, Conn PJ, Jones CK (2014) Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU152100. Neuropsychopharmacol 39:1578–1593
Cajina M, Nattini M, Song D, Smagin G, Jørgensen EB, Chandrasena G, Bundgaard C, Toft DB, Huang X, Acher F, Doller D (2013) Qualification of LSP1-2111 as a brain penetrant group III metabotropic glutamate receptor orthosteric agonist. ACS Med Chem Lett 5(2):119–123
Carbon M, Hsieh CH, Kane JM, Correll CU (2017) Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry 78(3):264–278
Conn PJ, Christopoulos A, Lindsley CW (2009b) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 8:41–54
Conn PJ, Lindsley CW, Jones CK (2009c) Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci 30:25–31
Corripio I, Ferreira A, Portella MJ, Pérez V, Escartí MJ, Del Valle Camacho M, Sauras RB, Alonso A, Grasa EM, Carrió I, Catafau AM, Alvarez E (2012) The role of striatal dopamine D2 receptors in the occurrence of extrapyramidal side effects: iodine-123-iodobenzamide single photon emission computed tomography study. Psychiatry Res 201:73–77
de Moura Linck V, Herrmann AP, Goerck GC, Iwu MM, Okunji CO, Leal MB, Elisabetsky E (2008) The putative antipsychotic alstonine reverses social interaction withdrawal in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 32:1449–1452
Dencker D, Weikop P, Sørensen G, Woldbye DP, Wörtwein G, Wess J, Fink-Jensen A (2012) An allosteric enhancer of M4 muscarinic acetylcholine receptor function inhibits behavioral and neurochemical effects of cocaine. Psychopharmacology 224:277–287
Ellaithy A, Younkin J, González-Maeso J, Logothetis DE (2015) Positive allosteric modulators of metabotropic glutamate 2 receptors in schizophrenia treatment. Trends Neurosci 38:506–516
Fervaha G, Agid O, Takeuchi H, Lee J, Foussias G, Zakzanis KK, Graff-Guerrero A, Remington G (2015) Extrapyramidal symptoms and cognitive test performance in patients with schizophrenia. Schizophr Res 161:351–356
Fervaha G, Caravaggio F, Mamo DC, Mulsant BH, Pollock BG, Nakajima S, Gerretsen P, Rajji TK, Mar W, Iwata Y, Plitman E, Chung JK, Remington G, Graff-Guerrero A (2016) Lack of association between dopaminergic antagonism and negative symptoms in schizophrenia: a positron emission tomography dopamine D2/3 receptor occupancy study. Psychopharmacology (Berlin) 233:3803–3813
Galloway CR, Lebois EP, Shagarabi SL, Hernandez NA, Manns JR (2014) Effects of selective activation of M1 and M4 muscarinic receptors on object recognition memory performance in rats. Pharmacology 93(1–2):57–64
Geyer MA, Ellenbroek B (2003) Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuro-Psychopharmacol Biol Psychiatry 27:1071–1079
Global Burden of Disease Study (2013), Collaborators (22 August 2015). Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 386: 743–800
Goudet C, Vilar B, Courtiol T, Deltheil T, Bessiron T, Brabet I, Oueslati N, Rigault D, Bertrand HO, McLean H, Daniel H, Amalric M, Acher F, Pin JP (2012) A novel selective metabotropic glutamate receptor 4 agonist reveals new possibilities for developing subtype selective ligands with therapeutic potential. FASEB J 26:1682–1693
Haracz JL (1982) The dopamine hypothesis: an overview of studies with schizophrenic patients. Schizophr Bull 8:438–469
Hashimoto K, Malchow B, Falkai P, Schmitt A (2013) Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur Arch Psychiatry Clin Neurosci 263:367–377
Heinz A, Schlagenhauf F (2010) Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull 36:472–485
Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI (1994) Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci 14:3351–3363
Ince E, Ciliax BJ, Levey AI (1997) Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse 27:357–366
Javitt DC (2004) Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry 9(11):984–997 979
Jeon J, Dencker D, Wörtwein G, Woldbye DP, Cui Y, Davis AA, Levey AI, Schütz G, Sager TN, Mørk A, Li C, Deng CX, Fink-Jensen A, Wess J (2010) A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J Neurosci 30:2396–2405
Kane JM, Mayerhoff D (1989) Do negative symptoms respond to pharmacological treatment? Br J Psychiatry Suppl 7:115–118
Kreitzer AC (2009) Physiology and pharmacology of striatal neurons. Annu Rev Neurosci 32:127–147
Kuroiwa M, Hamada M, Hieda E, Shuto T, Sotogaku N, Flajolet M, Snyder GL, Hendrick JP, Fienberg A, Nishi A (2012) Muscarinic receptors acting at pre- and post-synaptic sites differentially regulate dopamine/DARPP-32 signaling in striatonigral and striatopallidal neurons. Neuropharmacol 63:1248–1257
Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR (1991) Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci 10:3218–3226
Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ (1995) Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci 15:4077–4092
Lindsley CW, Stauffer SR (2013) Metabotropic glutamate receptor 5-positive allosteric modulators for the treatment of schizophrenia (2004–2012). Pharm Pat Anal 2:93–108
Loening AM, Gambhir SS (2003) AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2:131–137
McCormick DA, Connors BW, Lighthall JW, Prince DA (1985) Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J. Neurophysiol. 54:782–806.
Messer WS Jr (2002) Cholinergic agonists and the treatment of Alzheimer’s disease. Curr Top Med Chem 2:353–358
Moghaddam B, Jackson ME (2003) Glutamatergic animal models of schizophrenia. Ann N Y Acad Sci 1003:131–137
Moghaddam B, Javitt D (2012) From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacol 37:4–15
Muguruza C, Meana JJ, Callado LF (2016) Group II metabotropic glutamate receptors as targets for novel antipsychotic drugs. Front Pharmacol 7:130
Nadal L, Garcia N, Hurtado E, Simó A, Tomàs M, Lanuza MA, Santafé M, Tomàs J (2016) Presynaptic muscarinic acetylcholine autoreceptors (M1, M2 and M4 subtypes), adenosine receptors (A1 and A2A) and tropomyosin-related kinase B receptor (TrkB) modulate the developmental synapse elimination process at the neuromuscular junction. Mol Brain 9:67
Nicoletti F, Bruno V, Ngomba RT, Gradini R, Battaglia G (2015) Metabotropic glutamate receptors as drug targets: what's new? Curr Opin Pharmacol 20:89–94
Nilsson M, Hansson S, Carlsson A, Carlsson ML (2007) Differential effects of the N-methyl-d-aspartate receptor antagonist MK-801 on different stages of object recognition memory in mice. Neuroscience 149:123–130
Noda Y, Yamada K, Furukawa H, Nabeshima T (1995) Enhancement of immobility in a forced swimming test by subacute or repeated treatment with phencyclidine: a new model of schizophrenia. Br J Pharmacol 116:2531–2537
Noda Y, Mamiya T, Furukawa H, Nabeshima T (1997) Effects of antidepressants on phencyclidine-induced enhancement of immobility in a forced swimming test in mice. Eur J Pharmacol 324:135–140
Oh HK, Park SJ, Bae SG, Kim MJ, Jang JH, Ahn YJ, Woo H, Kwon G, Ryu JH (2013) Kami-ondam-tang, a traditional herbal prescription, attenuates the prepulse inhibition deficits and cognitive impairments induced by MK-801 in mice. J Ethnopharmacol 146:600–607
Ormel J, Petukhova M, Chatterji S, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Bromet EJ, Burger H, Demyttenaere K, de Girolamo G, Haro JM, Hwang I, Karam E, Kawakami N, Lépine JP, Medina-Mora ME, Posada-Villa J, Sampson N, Scott K, Ustün TB, Von Korff M, Williams DR, Zhang M, Kessler RC (2008) Disability and treatment of specific mental and physical disorders across the world. Br J Psychiatry 192:368–375
Pancani T, Bolarinwa C, Smith Y, Lindsley CW, Conn PJ, Xiang Z (2014) M4 mAChR-mediated modulation of glutamatergic transmission at corticostriatal synapses. ACS Chem Neurosci 5:318–324
Poels EM, Kegeles LS, Kantrowitz JT, Slifstein M, Javitt DC, Lieberman JA, Abi-Dargham A, Girgis RR (2014) Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Mol Psychiatry 19:20–29
Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B (2007) Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry 12:232–246
Rorick-Kehn LM, Johnson BG, Burkey JL, Wright RA, Calligaro DO, Marek GJ, Nisenbaum ES, Catlow JT, Kingston AE, Giera DD, Herin MF, Monn JA, McKinzie DL, Schoepp DD (2007) Pharmacological and pharmacokinetic properties of a structurally novel, potent, and selective metabotropic glutamate 2/3 receptor agonist: in vitro characterization of agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]-hexane-4,6-dicarboxylic acid (LY404039). J Pharmacol Exp Ther 321:308–317
Rössler W, Salize HJ, van Os J, Riecher-Rössler A (2005) Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol 15:399–409
Rubins DJ, Melega WP, Lacan G, Way B, Plenevaux A, Luxen A, Cherry SR (2003) Development and evaluation of an automated atlas-based image analysis method for microPET studies of the rat brain. Neuroimage 20:2100–2118
Schoepp DD, Jane DE, Monn JA (1999) Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacol 38:1431–1476
Schweinhardt P, Fransson P, Olson L, Spenger C, Andersson JL (2003) A template for spatial normalisation of MR images of the rat brain. J Neurosci Methods 129:105–113
Sladeczek F, Pin JP, Récasens M, Bockaert J, Weiss S (1985) Glutamate stimulates inositol phosphate formation in striatal neurones. Nature 317(6039):717–719
Sławińska A, Wierońska JM, Stachowicz K, Marciniak M, Łasoń-Tyburkiewicz M, Gruca P, Papp M, Kusek M, Tokarski K, Doller D, Pilc A (2013) The antipsychotic-like effects of positive allosteric modulators of metabotropic glutamate mGlu4 receptors in rodents. Br. J Pharmacol 169:1824–1839
Suratman S, Leach K, Sexton P, Felder C, Loiacono R, Christopoulos A (2011) Impact of species variability and 'probe-dependence' on the detection and in vivo validation of allosteric modulation at the M4 muscarinic acetylcholine receptor. Br J Pharmacol 162:1659–1662
Tantawy MN, Jones CK, Baldwin RM, Ansari MS, Conn PJ, Kessler RM, Peterson TE (2009) [(18)F]Fallypride dopamine D2 receptor studies using delayed microPET scans and a modified Logan plot. Nucl Med Biol 36:931–940
Tantawy MN, Peterson TE, Jones CK, Johnson K, Rook JM, Conn PJ, Baldwin RM, Ansari MS, Kessler RM (2011) Impact of isoflurane anesthesia on D2 receptor occupancy by [18F]fallypride measured by microPET with a modified Logan plot. Synapse 65:1173–1180
Tizabi Y, Russell LT, Johnson M, Darmani NA (2001) Nicotine attenuates DOI-induced head-twitch response in mice: implications for Tourette syndrome. Prog Neuro-Psychopharmacol Biol Psychiatry 25(7):1445–1457
van der Kloot W (1991) The regulation of quantal size. Prog Neurobiol 36:93–130
Velligan DI, Alphs L, Lancaster S, Morlock R, Mintz J (2009) Association between changes on the Negative Symptom Assessment scale (NSA-16) and measures of functional outcome in schizophrenia. Psychiatry Res 169:97–100
Vogel HG et al (2008) Drug discovery and evaluation: pharmacological assays. 3. Springer, Berlin, pp 580–581
Wierońska JM, Stachowicz K, Pałucha-Poniewiera A, Acher F, Brański P, Pilc A (2010) Metabotropic glutamate receptor 4 novel agonist LSP1-2111 with anxiolytic, but not antidepressant-like activity, mediated by serotonergic and GABAergic systems. Neuropharmacology 59:627–634
Wierońska JM, Stachowicz K, Acher F, Lech T, Pilc A (2012) Opposing efficacy of group III mGlu receptor activators, LSP1-2111 and AMN082, in animal models of positive symptoms of schizophrenia. Psychopharmacology (Berlin) 220:481–494
Wierońska JM, Sławińska A, Stachowicz K, Łasoń-Tyburkiewicz M, Gruca P, Papp M, Pilc A (2013) The reversal of cognitive, but not negative or positive symptoms of schizophrenia, by the mGlu2/3 receptor agonist, LY379268, is 5-HT1A dependent. Behav Brain Res 256:298–304
Wierońska JM, Sławińska A, Łasoń-Tyburkiewicz M, Gruca P, Papp M, Zorn SH, Doller D, Kłeczek N, Noworyta-Sokołowska K, Gołembiowska K, Pilc A (2015). The antipsychotic-like effects in rodents of the positive allosteric modulator Lu AF21934 involve 5-HT1A receptor signaling: mechanistic studies. Psychopharmacology (Berlin) 232: 259–273
Wierońska JM, Zorn SH, Doller D, Pilc A (2016) Metabotropic glutamate receptors as targets for new antipsychotic drugs: historical perspective and critical comparative assessment. Pharmacol Ther 157:10–27
Woźniak M, Acher F, Marciniak M, Lasoń-Tyburkiewicz M, Gruca P, Papp M, Pilc A, Wierońska JM (2016) Involvement of GABAB receptor signaling in antipsychotic-like action of the novel orthosteric agonist of the mGlu4 receptor, LSP4-2022. Curr Neuropharmacol 14:413–426
Woźniak M, Gołembiowska K, Noworyta-Sokołowska K, Acher F, Cieślik P, Kusek M et al (2017) Neurochemical and behavioral studies on the 5-HT1A-dependent antipsychotic action of the mGlu4 receptor agonist LSP4-2022. Neuropharmacol 115:149–165
Acknowledgements
This work was supported by grant no. 2015/17/B/NZ7/02984 (OPUS) from the National Science Center (NCN) in Poland to J. M. Wierońska.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Electronic supplementary material
ESM 1
(PDF 815 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cieślik, P., Woźniak, M., Rook, J.M. et al. Mutual activation of glutamatergic mGlu4 and muscarinic M4 receptors reverses schizophrenia-related changes in rodents. Psychopharmacology 235, 2897–2913 (2018). https://doi.org/10.1007/s00213-018-4980-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-4980-y