Abstract
Introduction
The use of second-generation antipsychotics (SGA) has been associated with metabolic changes. However, there are differences in the metabolic profile between SGAs. We have previously observed that ziprasidone had a more benign early metabolic profile compared to aripiprazole and quetiapine. However, a long-term follow-up is preferred to detect clinically relevant impairment in metabolic parameters. We aimed to compare the effect of aripiprazole, ziprasidone, and quetiapine on metabolic measures in first-episode non-affective psychosis patients after 1 year of treatment.
Material and methods
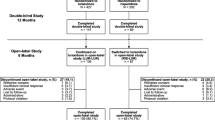
One hundred and sixty-five drug-naïve patients, suffering from a first episode of non-affective psychosis, were randomly assigned to receive quetiapine, ziprasidone, or aripiprazole. Weight and glycemic/lipid parameters were recorded at baseline and after 1 year of treatment.
Results
After 1 year of antipsychotic treatment, we found significant increments in weight, BMI, total cholesterol, LDL-cholesterol, triglycerides, and the triglyceride/HDL index in the sample as a whole. These changes produced a significant rise in the percentage of patients with obesity, hypercholesterolemia, and hypertriglyceridemia. However, when comparing the differential effect of each antipsychotic medication, we found no significant differences in any of the metabolic parameters between antipsychotics groups after 1 year of treatment.
Conclusion
We concluded that the antipsychotics studied present similar metabolic profiles. However, the primary exposure to SGAs during the first year of psychosis was associated with significant increases in weight and metabolic parameters, leading to increments in obesity, hypertriglyceridemia, and hypercholesterolemia.

Similar content being viewed by others
References
Alptekin K, Hafez J, Brook S et al (2009) Efficacy and tolerability of switching to ziprasidone from olanzapine, risperidone or haloperidol: an international, multicenter study. Int Clin Psychopharmacol 24:229–238
Andreasen N (1983) Scale for the assessment of negative symptoms (SANS). University of Iowa, Iowa City
Andreasen N (1984) Scale for the assessment of positive symptoms (SAPS). University of Iowa, Iowa City
Bonfioli E, Berti L, Goss C et al (2012) Health promotion lifestyle interventions for weight management in psychosis: a systematic review and meta-analysis of randomized controlled trials. BMC Psychiatry 12:78
Breier A, Berg PH, Thakore JH et al (2005) Olanzapine versus ziprasidone: results of a 28-week double-blind study in patients with schizophrenia. Am J Psychiatr 162:1879–1887
Carvalho AF, Sharma MS, Brunoni AR et al (2016) The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother. Psychosomatics 85(5):270–288
Chacón F, Mora F, Gervás-Ríos A et al (2011) Efficacy of lifestyle interventions in physical health management of patients with severe mental illness. Ann Gen Psychiatry 10:22
Chue P, Mandel FS, Therrien F (2014) The effect of ziprasidone on metabolic syndrome risk factors in subjects with schizophrenia: a 1 year, open-label, prospective study. Curr Med Res Opin 30(6):997–1005
Correll CU, Manu P, Olshanskiy V et al (2009) Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302:1765–1773
Correll CU, Robinson DG, Schooler NR et al (2014) Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry 71(12):1350–1363
Crespo-Facorro B, Ortiz-Garcia d l FV, Mata I et al (2013) Aripiprazole, ziprasidone and quetiapine in the treatment of first-episode non-affective psychosis: a 12-week randomized, flexible-dose, open-label trial. Schizophr Res 147:375–382
Crespo-Facorro B, de la Foz VO, Mata I et al (2014) Treatment of first-episode non-affective psychosis: a randomized comparison of aripiprazole, quetiapine and ziprasidone over 1 year. Psychopharmacology 231(2):357–366
Crespo-Facorro B, Pelayo-Teran JM, Mayoral-van Son J (2016) Current data on and clinical insights into the treatment of first episode nonaffective psychosis: a comprehensive review. Neurol Ther 5(2):105–130. https://doi.org/10.1007/s40120-016-0050-8
Daniel DG, Zimbroff DL, Potkin SG et al (1999) Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology 20:491–505
De Hert M, Detraux J, vanWinkel R et al (2011) Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 8:114–126
Foley DL and Morley KI (2011) Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry 68:609–616
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Grootens KP, van Veelen NM, Peuskens J et al (2011) Ziprasidone vs olanzapine in recent-onset schizophrenia and schizoaffective disorder: results of an 8-week double-blind randomized controlled trial. Schizophr Bull 37(2):352–361
Hjorthøj C, Stürup AE, McGrath JJ et al (2017) Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry 4(4):295–301
Jensen KG, Correll CU, Rudå D et al (2017) Pretreatment cardiometabolic status in youth with early-onset psychosis: baseline results from the TEA trial. J Clin Psychiatry. https://doi.org/10.4088/JCP.15m10479
Kahn RS, Fleischhacker WW, Boter H et al (2008) Effectiveness of antipsychotic drugsin first-episode schizophrenia and schizophreniform disorder: an openrandomised clinical trial. Lancet 371(9618):1085–1097
Kane JM, Carson WH, Saha AR et al (2002) Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 63:763–771
Karayal ON, Glue P, Bachinsky M et al (2011) Switching from quetiapine to ziprasidone: a sixteen-week, open-label, multicenter study evaluating the effectiveness and safety of ziprasidone in outpatient subjects with schizophrenia or schizoaffective disorder. J Psychiatr Pract 17(2):100–109
Kerwin R, Millet B, Herman E et al (2007) A multicentre, randomized, naturalistic, open-label study between aripiprazole and standard of care in the management of community-treated schizophrenic patients Schizophrenia Trial of Aripiprazole: (STAR) study. Eur Psychiatry 22:433–443
Kinon BJ, Lipkovich I, Edwards SB et al (2006) A 24-week randomized study of olanzapine versus ziprasidone in the treatment of schizophrenia or schizoaffective disorder in patients with prominent depressive symptoms. J Clin Psychopharmacol 26:157–162
Komossa K, Rummel-Kluge C, Hunger H et al (2009) Ziprasidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 4:CD006627
Lee SY, Park MH, Patkar AA et al (2011) A retrospective comparison of BMI changes and the potential risk factors among schizophrenic inpatients treated with aripiprazole, olanzapine, quetiapine or risperidone. Prog Neuropsychopharmacol Biol Psychiatry 35(2):490–496
Li Q, Chen D, Liu T et al (2016) Sex differences in body mass index and obesity in Chinese patients with chronic schizophrenia. J Clin Psychopharmacol 36(6):643–648
Lieberman JA, Stroup TS, McEvoy JP et al (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223
Lingjaerde O, Ahlfors UG, Bech P et al (1987) The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 334:1–100
Maayan L, Correll CU (2011) Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol 21(6):517–535
Mackin P, Waton T, Watkinson HM et al (2012) A four-year naturalistic prospective study of cardiometabolic disease in antipsychotic-treated patients. Eur Psychiatry 27(1):50–5
Malla A, Mustafa S, Rho A et al (2016) Therapeutic effectiveness and tolerability of aripiprazole as initial choice of treatment in first episode psychosis in an early intervention service: a one-year outcome study. Schizophr Res 174(1–3):120–125. https://doi.org/10.1016/j.schres.2016.04.036
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and betacell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
McEvoy JP, Daniel DG, Carson WH Jr et al (2007) A randomized, double-blind, placebo-controlled, study of the efficacy and safety of aripiprazole 10, 15 or 20 mg/day for the treatment of patients with acute exacerbations of schizophrenia. J Psychiatr Res 41:895–905
McLaughlin T, Reaven G, Abbasi F et al (2005) Is there a simple way to identify insulinresistant individuals at increased risk of cardiovascular disease? Am J Cardiol 96(3):399–404
Pappadopulos E, Newcomer JW, Kolluri S (2012) Changes in weight, plasma lipids, and glucose in adults treated with ziprasidone: a comprehensive analysis of Pfizer-initiated clinical trials. J Clin Psychiatry 73:e742–e748
Parabiaghi A, Tettamanti M, D’Avanzo B et al (2016) Metabolic syndrome and drug discontinuation in schizophrenia: a randomized trial comparing aripiprazole olanzapine and haloperidol. Acta Psychiatr Scand 133(1):63–75
Pelayo-Terán JM, Pérez-Iglesias R, Ramírez-Bonilla M et al (2008) Epidemiological factors associated with treated incidence of first-episode non-affective psychosis in Cantabria: insights from the Clinical Programme on Early Phases of Psychosis. Early Interv Psychiatry 2(3):178–187
Perez-Iglesias R, Crespo-Facorro B, Amado JA et al (2007) A 12-week randomized clinical trial to evaluate metabolic changes in drug-naive, first-episode psychosis patients treated with haloperidol, olanzapine, or risperidone. J Clin Psychiatry 68(11):1733–1740
Perez-Iglesias R, Crespo-Facorro B, Martinez-Garcia O et al (2008) Weight gain induced by haloperidol, risperidone and olanzapine after 1 year: findings of a randomized clinical trial in a drug-naïve population. Schizophr Res 99(1–3):13–22
Perez-Iglesias R, Mata I, Pelayo-Teran JM et al (2009) Glucose and lipid disturbances after 1 year of antipsychotic treatment in a drug-naïve population. Schizophr Res 107(2–3):115–121
Pérez-Iglesias R, Ortiz-Garcia de la Foz V, Martínez García O et al (2014a) Comparison of metabolic effects of aripiprazole, quetiapine and ziprasidone after 12 weeks of treatment in first treated episode of psychosis. Schizophr Res 159(1):90–94
Pérez-Iglesias R, Martínez-García O, Pardo-Garcia G et al (2014b) Course of weight gain and metabolic abnormalities in first treated episode of psychosis: the first year is a critical period for development of cardiovascular risk factors. Int J Neuropsychopharmacol 17(1):41–51
Pillinger T, Beck K, Gobjila C et al (2017) Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry 74(3):261–269
Rummel-Kluge C, Komossa K, Schwarz S et al (2010) Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 123:225–233
Spurling RD, Lamberti JS, Olsen D et al (2007) Changes in metabolic parameters with switching to aripiprazole from another second-generation antipsychotic: a retrospective chart review. J Clin Psychiatry 68:406–409
Stahl SM, Mignon L, Meyer JM (2009) Which comes first: atypical antipsychotic treatment or cardiometabolic risk? Acta Psychiatr Scand 119(3):171–9
Stroup TS, Lieberman JA, McEvoy JP et al (2006) Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatr 163:611–622
Stroup TS, McEvoy JP, Ring KD et al (2011) A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry 168:947–956
Takeuchi H, Uchida H, Suzuki T et al (2010) Changes in metabolic parameters following a switch to aripiprazole in Japanese patients with schizophrenia: one-year follow-up study. Psychiatry. Clin Neurosci 64:104–106
Zhai D, Lang Y, Feng Y et al (2017) Early onset of cardiometabolic risk factor profiles in drug naïve adolescents and young adults with first-episode schizophrenia. Schizophr Res pii: S0920-9964(17):30126–30123
Acknowledgements
This study was conducted as part of a clinical trial “Comparative Study of Aripiprazole, Quetiapine and Ziprasidone in the Treatment of First Episode Non-affective Psychosis (AZQ2005).” ClinicalTrials.gov Identifier: NCT02305823.
The authors wish to thank all “Programa Asistencial de las Fases Iniciales de Psicosis” (PAFIP) research team and all patients and family members who participated in the study.
Funding
The present study was carried out at the Hospital Marqués de Valdecilla, University of Cantabria, Santander, Spain, under the following grant support: Instituto de Salud Carlos III PI020499, PI050427, PI060507; Plan Nacional de Drogas Research Grant 2005-Orden sco/3246/2004; SENY Fundació Research Grant CI 2005–0308007; and Fundación Marqués de Valdecilla API07/011. Unrestricted educational and research grants from AstraZeneca, Pfizer, Bristol-Myers Squibb, and Johnson & Johnson provided support for PAFIP activities. No pharmaceutical industry or institutional sponsors participated in the study concept and design, data collection, analysis and interpretation of the results, and drafting the manuscript.
Author information
Authors and Affiliations
Contributions
BC-F designed the study and wrote the protocol. PSP evaluated the patients and collected the study variables. VO-G built and maintained the database and helped with the statistical analyses. RP-I and JV-B managed the literature searches. JV-B undertook the statistical analysis and wrote the first draft of the manuscript. ADM, RP-I, and BC-F contributed to the interpretation of the data and revised the manuscript critically. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Vázquez-Bourgon, J., Pérez-Iglesias, R., Ortiz-García de la Foz, V. et al. Long-term metabolic effects of aripiprazole, ziprasidone and quetiapine: a pragmatic clinical trial in drug-naïve patients with a first-episode of non-affective psychosis. Psychopharmacology 235, 245–255 (2018). https://doi.org/10.1007/s00213-017-4763-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4763-x