Abstract
Rationale
Strategies to reduce the misuse of mu opioid agonists are critically needed. Previous work has shown that kappa opioid agonists can diminish the abuse-related effects and augment the antinociceptive effects of mu agonists. However, use of traditional kappa agonists is limited by their dysphoric side effects.
Objectives
The current study examined the effects of nalfurafine, a clinically available atypical kappa agonist, on the reinforcing, thermal antinociceptive, and respiratory-depressant effects of oxycodone in male rats.
Methods
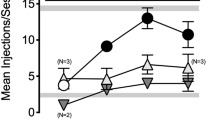
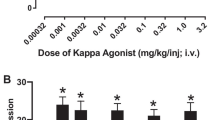
To determine oxycodone/nalfurafine mixture proportions to be examined intravenously across procedures, a progressive ratio (PR) self-administration procedure compared the reinforcing effects of oxycodone (56 μg/kg/inj) available alone or as a mixture with co-administered nalfurafine (0.32, 1, or 3.2 μg/kg/inj), corresponding to oxycodone/nalfurafine proportions of 175:1, 56:1, and 18:1, respectively. Next, PR and thermal antinociception dose-effect functions were each determined for oxycodone, nalfurafine, and the same oxycodone/nalfurafine mixture proportions. Finally, the respiratory-depressant effects of equi-antinociceptive doses of oxycodone, nalfurafine, and the mixtures were compared.
Results
Nalfurafine decreased the reinforcing effects of oxycodone, and the 18:1 mixture did not function as a reinforcer. Oxycodone and nalfurafine each produced dose-dependent antinociception, and the mixtures produced additive antinociception. In addition, antinociceptive doses of the 56:1 and 18:1 mixtures did not produce respiratory depression.
Conclusions
These results suggest that nalfurafine may augment the thermal antinociceptive effects while reducing the reinforcing and respiratory-depressant effects of oxycodone.




Similar content being viewed by others
References
Barrett AC (2006) Low efficacy opioids: implications for sex differences in opioid antinociception. Exp Clin Psychopharmacol 14:1–11
Bassi M, Nakamura NB, Furuya WI, Colombari DS, Menani JV, do Carmo JM, da Silva AA, Hall JE, Colombari E (2015) Activation of the brain melanocortin system is required for leptin-induced modulation of chemorespiratory function. Acta Physiol 213:893–901
Bolanos CA, Garmsen GM, Clair MA, McDougall SA (1996) Effects of the kappa-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developing rat. Eur J Pharmacol 317:1–8
Bruijnzeel AW (2009) Kappa-opioid receptor signaling and brain reward function. Brain Res Rev 62:127–146
Centers for disease control and prevention: National center for health statistics (2015) Number of age-adjusted rates of drug-poisoning deaths involving opioid analgesics and heroin: United States, 1999–2014. https://www.cdc.gov/nchs/data/health_policy/AADR_drug_poisoning_involving_OA_Heroin_US_2000-2014.pdf
Dogra S, Yadav PN (2015) Biased agonism at kappa opioid receptors: implication in pain and mood disorders. Eur J Pharmacol 763:184–190
Duthie DJ, Nimmo WS (1987) Adverse effects of opioid analgesic drugs. Br J Anaesth 59:61–77
Freeman KB, Naylor JE, Prisinzano TE, Woolverton WL (2014) Assessment of the kappa opioid agonist, salvinorin a, as a punisher of drug self-administration in monkeys. Psychopharmacology 231:2751–2758
Frenk SM, Porter KS, Paulozzi LJ (2015) Centers for disease control and prevention: National center for health statistics (2015) Prescription Opioid Analgesic Use Among Adults: United States, 1999–2012. https://www.cdc.gov/nchs/products/databriefs/db189.htm
Funada M, Suzuki T, Narita M, Misawa M, Nagase H (1993) Blockade of morphine reward through the activation of kappa-opioid receptors in mice. Neuropharmacology 32:1315–1323
Glick SD, Maisonneuve IM, Raucci AS (1995) Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res 681:147–152
Hasabe K, Kawai K, Suzuki T, Kawamura K, Tanaka T, Narita M et al (2004) Possible pharmacotherapy of the opioid kappa receptor agonist for drug dependence. Ann N Y Acad Sci 1025:404–413
Honma K, Hiroshige T (1978) Simultaneous determination of circadian rhythms of locomotor activity and body temperature in the rat. Jpn J Physiol 28:159–169
Huskinson SL, Naylor JE, Townsend EA, Rowlett JK, Blough BE, Freeman KB (2017) Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology 234:589–598
Inui S (2015) Nalfurafine hydrochloride to treat pruritus: a review. Clin Cosmet Investing Dermatol 8:249–255
Jones MR, Kaye AD, Kaye AJ, Urman RD (2016) The emerging therapeutic roles of kappa-opioid agonists. J Opioid Manag 12:101–107
Ko MC, Husbands SM (2009) Effects of atypical kappa-opioid receptor agonists on intrathecal morphine-induced itch and analgesia in primates. J Pharmacol Exp Ther 328:193–200
Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H (2010) Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant 25:1251–1257
Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H et al (2012) Efficacy and safety of a novel ĸ-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol 36:175–183
Kuzmin AV, Semenova S, Gerrits MA, Zvartau EE, Van Ree JM (1997) Kappa-opioid receptor agonist U50,488H modulates cocaine and morphine self-administration in drug naïve rats and mice. Eur J Pharmacol 321:265–271
Milan A (1973) Ventilation measured by body plethysmography in hibernating mammals and in poikitotherms. Respir Physiol 17:32–44
Millan MJ (1990) Kappa-opioid receptors and analgesia. Trends Pharmacol Sci 11:70–76
Mori T, Nomura M, Nagase H, Narita M, Suzuki T (2002) Effects of a newly synthesized kappa opioid receptor agonist, TRK-820, on the discriminative stimulus and rewarding effects of cocaine in rats. Psychopharmacology 161:17–22
National Research Council of the National Academies (2011). Guide for the Care and Use of Laboratory Animals. Eighth Edition. Washington, DC: The National Academies Press
Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D (2006) Preclinical assessment of candidate analgesic drugs: recent advancements and future challenges. J Pharmacol Exp Ther 319:507–514
Negus SS, Schrode K, Stevenson GW (2008) Mu/kappa opioid interactions in rhesus monkeys: implications for analgesia and abuse liability. Exp Clin Psychopharmacol 16:386–399
OxyContin® [package insert] (2016) Purdue Pharma LP. Stamford, CT, USA: December 2016
Schattaurer SS, Kuhar JR, Song A, Chavkin C (2017) Nalfurafine is a G-protein biased agonist having significantly greater bias at the human than rodent form of the kappa opioid receptor. Cell Signal 32:59–65
Shook JE, Watkins WD, Camporesi EM (1990) Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression. Am Rev Respir Dis 142:895–909
Tallarida RJ (2000) Drug synergism and dose-effect data analysis. Chapman and Hall/CRC, Boca Raton
Tallarida RJ (2012) Revisiting the isobole and related quantitative methods for assessing drug synergism. J Pharmacol Exp Ther 342:2–8
Thomsen M, Woldbye D, Wortwein G, Fink-Jensen A, Wess J, Caine SB (2005) Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci 25:8141–8149
Townsend EA, Beloate LN, Huskinson SL, Roma PG, Freeman KB (2015) Corn oil, but not cocaine, is a more effective reinforcer in obese than in lean Zucker rats. Physiol Behav 143:136–141
Ueno Y, Mori A, Yanagita T (2013) One year long-term study on the abuse-liability of nalfurafine in hemodialysis patients. Int J Clin Pharmacol Ther 51:823–831
United States food and drug administration center for drug evaluation and research (2015) Abuse-deterrent opioids evaluation and labeling guidance for industry. https://www.fda.gov/downloads/Drugs/Guidances/UCM334743.pdf
Acknowledgements
The authors would like to thank Hunter Bruce, Georganna Nutt, Yvonne Zuchowski, and Josh Woods for their expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was supported by R01-DA039167 to KBF from the National Institute on Drug Abuse.
Rights and permissions
About this article
Cite this article
Townsend, E.A., Naylor, J.E., Negus, S.S. et al. Effects of nalfurafine on the reinforcing, thermal antinociceptive, and respiratory-depressant effects of oxycodone: modeling an abuse-deterrent opioid analgesic in rats. Psychopharmacology 234, 2597–2605 (2017). https://doi.org/10.1007/s00213-017-4652-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4652-3