Abstract
Objective
Here, we present the data indicating that chronic treatment with fluoxetine regulates Cav-1/PTEN/PI3K/AKT/GSK-3β signalling pathway and glycogen content in primary cultures of astrocytes with bi-phasic concentration dependence.
Results
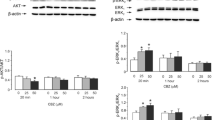
At lower concentrations, fluoxetine downregulates gene expression of Cav-1, decreases membrane content of PTEN, increases activity of PI3K/AKT, and elevates GSK-3β phosphorylation thus suppressing its activity. At higher concentrations, fluoxetine acts in an inverse fashion. As expected, fluoxetine at lower concentrations increased while at higher concentrations decreased glycogen content in astrocytes.
Conclusions
Our findings indicate that bi-phasic regulation of glycogen content via Cav-1/PTEN/PI3K/AKT/GSK-3β pathway by fluoxetine may be responsible for both therapeutic and side effects of the drug.





Similar content being viewed by others
References
Barley K, Dracheva S, Byne W (2009) Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res 112:54–64
Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ (2011) Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 16:634–646
Bolo NR, Hodé Y, Nédélec JF, Lainé E, Wagner G, Macher JP (2000) Brain pharmacokinetics and tissue distribution in vivo of fluvoxamine and fluoxetine by fluorine magnetic resonance spectroscopy. Neuropsychopharmacology 23:428–438
Chidlow JH Jr, Sessa WC (2010) Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res 86:219–225
Diniz BS, Talib LL, Joaquim HP, de Paula VR, Gattaz WF, Forlenza OV (2011) Platelet GSK3B activity in patients with late-life depression: marker of depressive episode severity and cognitive impairment? World J Biol Psychiatry 12:216–222
Duran J, Saez I, Gruart A, Guinovart JJ, Delgado-García JM (2013) Impairment in long-term memory formation and learning-dependent synaptic plasticity in mice lacking glycogen synthase in the brain. J Cereb Blood Flow Metab 33:550–556
Fang X, Yu SX, Lu Y, Bast RC Jr, Woodgett JR, Mills GB (2000) Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A 97:11960–11965
Gao V, Suzuki A, Magistretti PJ, Lengacher S, Pollonini G, Steinman MQ, Alberini CM (2016) Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc Natl Acad Sci U S A 113:8526–8531
Gibbs ME, Hertz L (2014) Serotonin mediation of early memory formation via 5-HT2B receptor-induced glycogenolysis in the day-old chick. Front Pharmacol 5:54
Gibbs ME, Hutchinson DS (2012) Rapid turnover of glycogen in memory formation. Neurochem Res 37:2456–2463
Gould TD, Manji HK (2005) Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology 30:1223–1237
Henry ME, Schmidt ME, Hennen J, Villafuerte RA, Butman ML, Tran P, Kerner LT, Cohen B, Renshaw PF (2005) A comparison of brain and serum pharmacokinetics of R-fluoxetine and racemic fluoxetine: a 19-F MRS study. Neuropsychopharmacology 30:1576–1583
Hertz L, Bender AS, Woodbury DM, White HS (1989) Potassium-stimulated calcium uptake in astrocytes and its potent inhibition by nimodipine. J Neurosci Res 22:209–215
Hertz L, Juurlink BHJ, Szuchet S (1985) Cell cultures. In: Lajtha A (ed) Handbook of neurochemistry. Plenum Press, New York, pp 603–661
Hertz L, Peng L, Lai JC (1998) Functional studies in cultured astrocytes. Methods 16:293–310
Hertz L, Schousboe A, Boechler N, Mukerji S, Fedoroff S (1978) Kinetic characteristics of the glutamate uptake into normal astrocytes in cultures. Neurochem Res 3:1–14
Hertz L, Xu J, Song D, Du T, Li B, Yan E, Peng L (2015) Astrocytic glycogenolysis: mechanisms and functions. Metab Brain Dis 30:317–333
Hertz L, Xu J, Song D, Du T, Yan E, Peng L (2013) Brain glycogenolysis, adrenoceptors, pyruvate carboxylase, Na+,K+-ATPase and Marie E. Gibbs’ pioneering learning studies. Front Integr Neurosci 7:20
Ibrahim MZ (1975) Glycogen and its related enzymes of metabolism in the central nervous system. Adv Anat Embryol Cell Biol 52:3–89
Joaquim HP, Talib LL, Forlenza OV, Diniz BS, Gattaz WF (2012) Long-term sertraline treatment increases expression and decreases phosphorylation of glycogen synthase kinase-3B in platelets of patients with late-life major depression. J Psychiatr Res 46:1053–1058
Karege F, Perroud N, Burkhardt S, Fernandez R, Ballmann E, La Harpe R, Malafosse A (2012) Protein levels of β-catenin and activation state of glycogen synthase kinase-3β in major depression. A study with postmortem prefrontal cortex J Affect Disord 136:185–188
Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, Turecki G (2009) Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Mol Psychiatry 14:175–189
Kong EK, Peng L, Chen Y, Yu AC, Hertz L (2002) Up-regulation of 5-HT2B receptor density and receptor-mediated glycogenolysis in mouse astrocytes by long-term fluoxetine administration. Neurochem Res 27:113–120
Li B, Zhang S, Zhang H, Nu W, Cai L, Hertz L, Peng L (2008) Fluoxetine-mediated 5-HT2B receptor stimulation in astrocytes causes EGF receptor transactivation and ERK phosphorylation. Psychopharmacology 201:443–458
Liu S, Sun N, Xu Y, Yang C, Ren Y, Liu Z, Cao X, Sun Y, Xu Q, Zhang K, Shen Y (2012) Possible association of the GSK3β gene with the anxiety symptoms of major depressive disorder and P300 waveform. Genet Test Mol Biomarkers 16:1382–1389
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
McNally L, Bhagwagar Z, Hannestad J (2008) Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr 13:501–510
Meier E, Hertz L, Schousboe A (1991) Neurotransmitters as developmental signals. Neurochem Int 19:1–15
O’Dowd BS, Barrington J, Ng KT, Hertz E, Hertz L (1995) Glycogenolytic response of primary chick and mouse cultures of astrocytes to noradrenaline across development. Brain Res Dev Brain Res 88:220–223
O’Dowd BS, Gibbs ME, Ng KT, Hertz E, Hertz L (1994) Astrocytic glycogenolysis energizes memory processes in neonate chicks. Brain Res Dev Brain Res 78:137–141
Peng L, Martin-vasallo P, Sweadner KJ (1997) Isoforms of Na, K-ATPase alpha and beta subunits in the rat cerebellum and in granule cell cultures. J Neurosci 17:3488–3502
Perez-Costas E, Gandy JC, Melendez-Ferro M, Roberts RC, Bijur GN (2010) Light and electron microscopy study of glycogen synthase kinase-3beta in the mouse brain. PLoS One 5:e8911
Phukan S, Babu VS, Kannoji A, Hariharan R, Balaji VN (2010) GSK3beta: role in therapeutic landscape and development of modulators. Br J Pharmacol 160:1–19
Pláteník J, Fišar Z, Buchal R, Jirák R, Kitzlerová E, Zvěřová M, Raboch J (2014) GSK3β, CREB, and BDNF in peripheral blood of patients with Alzheimer’s disease and depression. Prog Neuro-Psychopharmacol Biol Psychiatry 50:83–93
Popoli M, Yan Z, McEwen BS, Sanacora G (2011) The stressed synapse: the impact ofstress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13:22–37
Rajkowska G, Stockmeier CA (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14:1225–1236
Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP (2001) Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 276:38121–38138
Ren J, Song D, Bai Q, Verkhratsky A, Peng L (2015) Fluoxetine induces alkalinization of astroglial cytosol through stimulation of sodium-hydrogen exchanger 1: dissection of intracellular signaling pathways. Front Cell Neurosci 9:61
Robbins HL, Hague A (2016) The PI3K/Akt pathway in tumors of endocrine tissues. Front Endocrinol (Lausanne) 6:188
Sanacora G, Banasr M (2013) From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry 73:1172–1179
Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, Rehal S, Klempan T, Gratton A, Benkelfat C, Rouleau GA, Mechawar N, Turecki G (2009) Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One 4:e6585
Shin T, Kim H, Jin JK, Moon C, Ahn M, Tanuma N, Matsumoto Y (2005) Expression of caveolin-1, -2, and -3 in the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis. J Neuroimmunol 165:11–20
Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP (1999) Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol 19:7289–7304
Sowa G (2011) Novel insights into the role of caveolin-2 in cell- and tissue-specific signaling and function. Biochem Res Int 2011:809259
Stary CM, Tsutsumi YM, Patel PM, Head BP, Patel HH, Roth DM (2012) Caveolins: targeting pro-survival signaling in the heart and brain. Front Physiol 3:393
Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144:810–823
Szymańska M, Suska A, Budziszewska B, Jaworska-Feil L, Basta-Kaim A, Leśkiewicz M, Kubera M, Gergont A, Kroczka S, Kaciński M, Lasoń W (2009) Prenatal stress decreases glycogen synthase kinase-3 phosphorylation in the rat frontal cortex. Pharmacol Rep 61:612–620
Verkhratsky A, Butt AM (2013) Glial physiology and pathophysiology. Wiley-Blackwell, Chichester
Verkhratsky A, Rodríguez JJ, Steardo L (2014) Astrogliopathology: a central element of neuropsychiatric diseases? Neuroscientist 20:576–588
Virgintino D, Robertson D, Errede M, Benagiano V, Tauer U, Roncali L, Bertossi M (2002) Expression of caveolin-1 in human brain microvessels. Neuroscience 115:145–152
Wang W, Gu L, Verkhratsky A, Peng L (2016) Ammonium increases TRPC1 expression via Cav-1/PTEN/AKT/GSK3β pathway. Neurochem Res. doi:10.1007/s11064-016-2004-z
Xia H, Khalil W, Kahm J, Jessurun J, Kleidon J, Henke CA (2010) Pathologic caveolin-1 regulation of PTEN in idiopathic pulmonary fibrosis. Am J Pathol 176:2626–2637
Xu J, Song D, Xue Z, Gu L, Hertz L, Peng L (2013) Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: potential implications for K+ homeostasis and glycogen usage in brain. Neurochem Res 38:472–485
Zhang K, Song X, Xu Y, Li X, Liu P, Sun N, Zhao X, Liu Z, Xie Z, Peng J (2013) Continuous GSK-3β overexpression in the hippocampal dentate gyrus induces prodepressant-like effects and increases sensitivity to chronic mild stress in mice. J Affect Disord 146:45–52
Zschocke J, Manthey D, Bayatti N, van der Burg B, Goodenough S, Behl C (2002) Estrogen receptor alpha-mediated silencing of caveolin gene expression in neuronal cells. J Biol Chem 277:38772–38780
Acknowledgements
This study was supported by Grant No. 31400925 to DS from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
QB and DS contributed equally to this article.
Rights and permissions
About this article
Cite this article
Bai, Q., Song, D., Gu, L. et al. Bi-phasic regulation of glycogen content in astrocytes via Cav-1/PTEN/PI3K/AKT/GSK-3β pathway by fluoxetine. Psychopharmacology 234, 1069–1077 (2017). https://doi.org/10.1007/s00213-017-4547-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4547-3