Abstract
Rationale
We previously reported that the fluvoxamine-induced increase in prefrontal dopamine levels is enhanced by adrenalectomy/castration (which results in circulating neurosteroid deficiency), via combined activation of serotonin1A (5-HT1A) and σ1 receptors. However, the mechanistic details of the interaction between 5-HT1A and σ1 receptors are unknown.
Objectives
Because most neurosteroids have affinity for γ-aminobutyric acid (GABA)A receptors, in the present study, we examined the involvement of GABAA receptors in this process.
Results
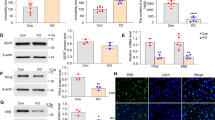
Adrenalectomy/castration decreased pentobarbital-induced sleeping time in mice, suggesting that it reduced GABAA receptor function. The GABAA receptor antagonist picrotoxin (1 mg/kg) enhanced the fluvoxamine-induced increase in prefrontal dopamine, but not noradrenaline or serotonin, levels in mice, suggesting that picrotoxin mimicked the effect of adrenalectomy/castration. Picrotoxin also potentiated the increase in prefrontal dopamine levels mediated by co-administration of the 5-HT1A receptor agonist osemozotan and the σ1 receptor agonist (+)-SKF-10,047, while it did not affect the co-administration-induced changes in noradrenaline and serotonin levels. Conversely, the GABAA receptor agonist diazepam (1 mg/kg) blocked the effect of adrenalectomy/castration on the fluvoxamine-induced increase in prefrontal dopamine levels. Co-administration of osemozotan and (+)-SKF-10,047 did not affect the expression of the neuronal activity marker c-Fos in the prefrontal cortex, ventral tegmental area, and nucleus accumbens in control mice, while it increased the c-Fos expression only in the prefrontal cortex and ventral tegmental area in picrotoxin-treated mice.
Conclusions
These results suggest that the GABAA receptor plays a key role in mediating the synergistic effects of 5-HT1A and σ1 receptor activation on prefrontal dopamine neurotransmission.








Similar content being viewed by others
References
Ago Y, Nakamura S, Uda M, Kajii Y, Abe M, Baba A, Matsuda T (2006) Attenuation by the 5-HT1A receptor agonist osemozotan of the behavioral effects of single and repeated methamphetamine in mice. Neuropharmacology 51:914–922. doi:10.1016/j.neuropharm.2006.06.001
Ago Y, Yano K, Hiramatsu N, Takuma K, Matsuda T (2011) Fluvoxamine enhances prefrontal dopaminergic neurotransmission in adrenalectomized/castrated mice via both 5-HT reuptake inhibition and σ1 receptor activation. Psychopharmacology 217:377–386. doi:10.1007/s00213-011-2293-5
Ago Y, Araki R, Tanaka T, Sasaga A, Nishiyama S, Takuma K, Matsuda T (2013) Role of social encounter-induced activation of prefrontal serotonergic systems in the abnormal behaviors of isolation-reared mice. Neuropsychopharmacology 38:1535–1547. doi:10.1038/npp.2013.52
Ago Y, Hasebe S, Nishiyama S, Oka S, Onaka Y, Hashimoto H, Takuma K, Matsuda T (2015) The female encounter test: a novel method for evaluating reward-seeking behavior or motivation in mice. Int J Neuropsychopharmacol 18:pyv062. doi:10.1093/ijnp/pyv062
Araki R, Ago Y, Hasebe S, Nishiyama S, Tanaka T, Oka S, Takuma K, Matsuda T (2014) Involvement of prefrontal AMPA receptors in encounter stimulation-induced hyperactivity in isolation-reared mice. Int J Neuropsychopharmacol 17:883–893. doi:10.1017/S1461145713001582
Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L (2015) Circuit architecture of VTA dopamine neurons revealed by systematic input–output mapping. Cell 162:622–634. doi:10.1016/j.cell.2015.07.015
Belelli D, Lambert JJ (2005) Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci 6:565–575. doi:10.1038/nrn1703
Carr DB, Sesack SR (2000) Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci 20:3864–3873
Carver CM, Reddy DS (2013) Neurosteroid interactions with synaptic and extrasynaptic GABAA receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology 230:151–188. doi:10.1007/s00213-013-3276-5
Chiodo LA, Bunney BS (1983) Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci 3:1607–1619
Compagnone NA, Mellon SH (2000) Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol 21:1–56. doi:10.1006/frne.1999.0188
Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ (1991) Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cereb Cortex 1:273–292. doi:10.1093/cercor/1.4.273
Egashira N, Harada S, Okuno R, Matsushita M, Nishimura R, Mishima K, Iwasaki K, Orito K, Fujiwara M (2007) Involvement of the sigma1 receptor in inhibiting activity of fluvoxamine on marble-burying behavior: comparison with paroxetine. Eur J Pharmacol 563:149–154. doi:10.1016/j.ejphar.2007.02.019
Eser D, Schüle C, Baghai TC, Romeo E, Rupprecht R (2006) Neuroactive steroids in depression and anxiety disorders: clinical studies. Neuroendocrinology 84:244–254. doi:10.1159/000097879
Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic Press, Inc., San Diego
Garzón M, Pickel VM (2004) Ultrastructural localization of Leu5-enkephalin immunoreactivity in mesocortical neurons and their input terminals in rat ventral tegmental area. Synapse 52:38–52. doi:10.1002/syn.20000
Girish C, Raj V, Arya J, Balakrishnan S (2013) Involvement of the GABAergic system in the anxiolytic-like effect of the flavonoid ellagic acid in mice. Eur J Pharmacol 710:49–58. doi:10.1016/j.ejphar.2013.04.003
Hasebe S, Hiramatsu N, Ago Y, Mori K, Watabe Y, Hashimoto H, Takuma K, Matsuda T (2015) Role of the 5-HT1A autoreceptor in the enhancement of fluvoxamine-induced increases in prefrontal dopamine release by adrenalectomy/castration in mice. J Pharmacol Sci 127:232–235. doi:10.1016/j.jphs.2015.01.001
Hashimoto K, Fujita Y, Iyo M (2007) Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacology 32:514–521. doi:10.1038/sj.npp.1301047
Hayashi T, Su TP (2008) An update on the development of drugs for neuropsychiatric disorders: focusing on the σ1 receptor ligand. Expert Opin Ther Targets 12:45–58. doi:10.1517/14728222.12.1.45
Hiramatsu N, Ago Y, Hasebe S, Nishimura A, Mori K, Takuma K, Matsuda T (2013) Synergistic effect of 5-HT1A and σ1 receptor activation on prefrontal dopaminergic transmission under circulating steroid deficiency. Neuropharmacology 75:53–61. doi:10.1016/j.neuropharm.2013.06.026
Ishikawa M, Ishiwata K, Ishii K, Kimura Y, Sakata M, Naganawa M, Oda K, Miyatake R, Fujisaki M, Shimizu E, Shirayama Y, Iyo M, Hashimoto K (2007) High occupancy of sigma-1 receptors in the human brain after single oral administration of fluvoxamine: a positron emission tomography study using [11C]SA4503. Biol Psychiatry 62:878–883. doi:10.1016/j.biopsych.2007.04.001
Ishima T, Fujita Y, Hashimoto K (2014) Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells. Eur J Pharmacol 727:167–173. doi:10.1016/j.ejphar.2014.01.064
Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T (2010) Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice. J Neurochem 114:259–270. doi:10.1111/j.1471-4159.2010.06750.x
Kovács KJ (2008) Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol 20:665–672. doi:10.1111/j.1365-2826.2008.01734.x
Luscher B, Shen Q, Sahir N (2011) The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 16:383–406. doi:10.1038/mp.2010.120
Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL (2006) k opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A 103:2938–2942. doi:10.1073/pnas.0511159103
Matsumoto K, Ojima K, Watanabe H (1996) Neurosteroidal modulation of social isolation-induced decrease in pentobarbital sleep in mice. Brain Res 708:1–6. doi:10.1016/0006-8993(95)01277-X
Maurice T, Urani A, Phan VL, Romieu P (2001) The interaction between neuroactive steroids and the σ1 receptor function: behavioral consequences and therapeutic opportunities. Brain Res Brain Res Rev 37:116–132. doi:10.1016/S0165-0173(01)00112-6
National Research Council (1996) Guide for the Care and Use of Laboratory Animals, National Academy Press: Washington, DC.
Nishimura T, Ishima T, Iyo M, Hashimoto K (2008) Potentiation of nerve growth factor-induced neurite outgrowth by fluvoxamine: role of sigma-1 receptors, IP3 receptors and cellular signaling pathways. PLoS ONE 3:e2558. doi:10.1371/journal.pone.0002558
Phan VL, Su TP, Privat A, Maurice T (1999) Modulation of steroidal levels by adrenalectomy/castration and inhibition of neurosteroid synthesis enzymes affect σ1 receptor-mediated behaviour in mice. Eur J Neurosci 11:2385–2396. doi:10.1046/j.1460-9568.1999.00656.x
Redrobe JP, Bourin M (1998) Dose-dependent influence of buspirone on the activities of selective serotonin reuptake inhibitors in the mouse forced swimming test. Psychopharmacology 138:198–206. doi:10.1007/s002130050663
Renard CE, Fiocco AJ, Clenet F, Hascoet M, Bourin M (2001) Is dopamine implicated in the antidepressant-like effects of selective serotonin reuptake inhibitors in the mouse forced swimming test? Psychopharmacology 159:42–50. doi:10.1007/s002130100836
Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio G (2000) Social isolation-induced decreases in both the abundance of neuroactive steroids and GABAA receptor function in rat brain. J Neurochem 75:732–740. doi:10.1046/j.1471-4159.2000.0750732.x
Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI (1998) Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci 18:2697–2708
Swanson LW (1982) The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull 9:321–353. doi:10.1016/0361-9230(82)90145-9
Urani A, Roman FJ, Phan VL, Su TP, Maurice T (2001) The antidepressant-like effect induced by σ1-receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. J Pharmacol Exp Ther 298:1269–1279
Uzunova V, Sampson L, Uzunov DP (2006) Relevance of endogenous 3α-reduced neurosteroids to depression and antidepressant action. Psychopharmacology 186:351–361. doi:10.1007/s00213-005-0201-6
Acknowledgments
This study was supported in part by JSPS KAKENHI (25460099 (YA), 26293020 (HH), 26670122 (HH), 15H01288 (HH), and 15 K18874 (YO)), the Neuropsychiatry Drug Discovery Consortium established by Dainippon Sumitomo Pharma Co., Ltd. (Japan) with Osaka University (TM, HH), Takeda Science Foundation (Japan) (YA), Research Foundation for Pharmaceutical Sciences (Japan) (YA), and the Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers (Grant No. S2603) (HH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experimental procedures were conducted in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Yukio Ago, Shigeru Hasebe and Naoki Hiramatsu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ago, Y., Hasebe, S., Hiramatsu, N. et al. Involvement of GABAA receptors in 5-HT1A and σ1 receptor synergism on prefrontal dopaminergic transmission under circulating neurosteroid deficiency. Psychopharmacology 233, 3125–3134 (2016). https://doi.org/10.1007/s00213-016-4353-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4353-3