Abstract
Rationale
The selective serotonin reuptake inhibitor (SSRI) fluoxetine is the only psychopharmacological agent approved for use in children. While short-term studies of side effects have been performed, long-term consequences for brain development are not known. Such studies can be performed in appropriate animal models if doses modeling therapeutic use in children are known.
Objectives
The goal of this study was to identify a daily dose of fluoxetine in juvenile monkeys which would result in serum fluoxetine and norfluoxetine concentrations in the therapeutic range for children.
Methods
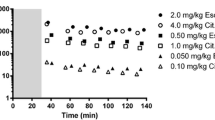
Juvenile (2.5-year-old rhesus monkeys, n = 6) received single administration of doses of 1, 2, and 4 mg/kg day fluoxetine on a background of chronic dosing at an intermediate level to provide steady-state conditions to model therapeutic administration. Using nonlinear modeling, standard pharmacokinetic parameters were derived. Cerebrospinal fluid monoamine neurotransmitters were assayed to confirm the pharmacological effects.
Results
Dose-dependent area under the curve (AUC) and C max values were seen, while T max and absorption/elimination half-lives were minimally influenced by dose. A dosage of 2 mg/kg day given over a 14-week period led to steady-state serum concentrations of active agent (fluoxetine + norfluoxetine) similar to those recorded from drug monitoring studies in children. Cisternal cerebrospinal fluid concentrations of serotonin increased significantly over the 14-week period, while concentrations of the serotonin metabolite (5-HIAA) were lower but not significantly different.
Conclusions
A dose of 2 mg/kg day fluoxetine in juvenile rhesus monkeys provides an internal dose similar to therapeutic use in children and will help establish a valuable animal model for understanding fluoxetine’s therapeutic and potential adverse effects in children.



Similar content being viewed by others
References
Alfaro CL, Lam YW, Simpson J, Ereshefsky L (2000) CYP2D6 inhibition by fluoxetine, paroxetine, sertraline, and venlafaxine in a crossover study: intraindividual variability and plasma concentration correlations. J Clin Pharmacol 40:58–66
Anderson GM (2004) Peripheral and central neurochemical effects of the selective serotonin reuptake inhibitors (SSRIs) in humans and nonhuman primates: assessing bioeffect and mechanisms of action. Int J Dev Neurosci 22:397–404
Anderson GM, Bennett AJ, Weld KP, Pushkas JG, Ocame DM, Higley JD (2002) Serotonin in cisternal cerebrospinal fluid of rhesus monkeys: basal levels and effects of sertraline administration. Psychopharmacology 161:95–99
Anderson GM, Barr CS, Lindell S, Durham AC, Shifrovich I, Higley JD (2005) Time course of the effects of the serotonin-selective reuptake inhibitor sertraline on central and peripheral serotonin neurochemistry in the rhesus monkey. Psychopharmacology 178:339–346
Bergstrom RF, Lemberger L, Farid NA, Wolen RL (1988) Clinical pharmacology and pharmacokinetics of fluoxetine: a review. Br J Psychiatry Suppl 153:47–50
Blardi P, de Lalla A, Auteri A, Iapichino S, Dell'Erba A, Castrogiovanni P (2005) Plasma catecholamine levels after fluoxetine treatment in depressive patients. Neuropsychobiology 51:72–76
Blazquez A, Mas S, Plana MT, Lafuente A, Lazaro L (2012) Fluoxetine pharmacogenetics in child and adult populations. Eur Child Adolesc Psychiatry 21:599–610
Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW (2002) Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology (Berl) 160:353–361
Chu S, Metcalfe CD (2007) Analysis of paroxetine, fluoxetine and norfluoxetine in fish tissues using pressurized liquid extraction, mixed mode solid phase extraction cleanup and liquid chromatography-tandem mass spectrometry. J Chromatogr A 1163:112–118
Clarke AS, Kraemer GW, Kupfer DJ (1998) Effects of rearing condition on HPA axis response to fluoxetine and desipramine treatment over repeated social separations in young rhesus monkeys. Psychiatry Res 79:91–104
Clarke AS, Ebert MH, Schmidt DE, McKinney WT, Kraemer GW (1999) Biogenic amine activity in response to fluoxetine and desipramine in differentially reared rhesus monkeys. Biol Psychiatry 46:221–228
De Bellis MD, Geracioti TD Jr, Altemus M, Kling MA (1993) Cerebrospinal fluid monoamine metabolites in fluoxetine-treated patients with major depression and in healthy volunteers. Biol Psychiatry 33:636–641
Ereshefsky L, Riesenman C, Lam YW (1996) Serotonin selective reuptake inhibitor drug interactions and the cytochrome P450 system. J Clin Psychiatry 57(Suppl 8):17–24, discussion 25
FDA (2003) Approval letter: application number 18-936/SE5-064. Center for Drug Evaluation and Research, Food and Drug Administration
Fontenot MB, Padgett EE 3rd, Dupuy AM, Lynch CR, De Petrillo PB, Higley JD (2005) The effects of fluoxetine and buspirone on self-injurious and stereotypic behavior in adult male rhesus macaques. Comp Med 55:67–74
Fontenot MB, Musso MW, McFatter RM, Anderson GM (2009) Dose-finding study of fluoxetine and venlafaxine for the treatment of self-injurious and stereotypic behavior in rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 48:176–184
Golub MS, Hogrefe CE, Unger EL (2012) Influence of prenatal iron deficiency and MAOA genotype on response to social challenge in rhesus monkey infants. Genes Brain Behav 11:278–290
Hiemke C, Hartter S (2000) Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther 85:11–28
Kim H, Lim SW, Kim S, Kim JW, Chang YH, Carroll BJ, Kim DK (2006) Monoamine transporter gene polymorphisms and antidepressant response in Koreans with late-life depression. JAMA 296:1609–1618
Koch S, Perry KW, Nelson DL, Conway RG, Threlkeld PG, Bymaster FP (2002) R-fluoxetine increases extracellular DA, NE, as well as 5-HT in rat prefrontal cortex and hypothalamus: an in vivo microdialysis and receptor binding study. Neuropsychopharmacology 27:949–959
Koelch M, Pfalzer AK, Kliegl K, Rothenhofer S, Ludolph AG, Fegert JM, Burger R, Mehler-Wex C, Stingl J, Taurines R, Egberts K, Gerlach M (2012) Therapeutic drug monitoring of children and adolescents treated with fluoxetine. Pharmacopsychiatry 45:72–76
Laudenslager ML, Clarke AS (2000) Antidepressant treatment during social challenge prior to 1 year of age affects immune and endocrine responses in adult macaques. Psychiatry Res 95:25–34
Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A (1997) The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm 104:1259–1266
Li Y, Emm T, Yeleswaram S (2011) Simultaneous determination of fluoxetine and its major active metabolite norfluoxetine in human plasma by LC-MS/MS using supported liquid extraction. Biomed Chromatogr 11:1245–1251
Lundmark J, Walinder J, Alling C, Manniche PM, Dalgaard L (1994) The effect of paroxetine on cerebrospinal fluid concentrations of neurotransmitter metabolites in depressed patients. Eur Neuropsychopharmacol 4:1–6
Martensson B, Nyberg S, Toresson G, Brodin E, Bertilsson L (1989) Fluoxetine treatment of depression. Clinical effects, drug concentrations and monoamine metabolites and N-terminally extended substance P in cerebrospinal fluid. Acta Psychiatr Scand 79:586–596
Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP (2005) Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry 57:167–172
Qu Y, Aluisio L, Lord B, Boggs J, Hoey K, Mazur C, Lovenberg T (2009) Pharmacokinetics and pharmacodynamics of norfluoxetine in rats: increasing extracellular serotonin level in the frontal cortex. Pharmacol Biochem Behav 92:469–473
Sawyer EK, Howell LL (2011) Pharmacokinetics of fluoxetine in rhesus macaques following multiple routes of administration. Pharmacology 88:44–49
Sheline Y, Bardgett ME, Csernansky JG (1997) Correlated reductions in cerebrospinal fluid 5-HIAA and MHPG concentrations after treatment with selective serotonin reuptake inhibitors. J Clin Psychopharmacol 17:11–14
Shrestha SS, Nelson EE, Liow JS, Gladding R, Lyoo CH, Noble PL, Morse C, Henter ID, Kruger J, Zhang B, Suomi SJ, Svenningsson P, Pike VW, Winslow JT, Leibenluft E, Pine DS, Innis RB (2014) Fluoxetine administered to juvenile monkeys: effects on the serotonin transporter and behavior. Am J Psychiatry. doi:10.1176/appi.ajp.2013.13020183
Silva H, Iturra P, Solari A, Villarroel J, Jerez S, Jimenez M, Galleguillos F, Bustamante ML (2010) Fluoxetine response in impulsive-aggressive behavior and serotonin transporter polymorphism in personality disorder. Psychiatr Genet 20:25–30
Wilens TE, Cohen L, Biederman J, Abrams A, Neft D, Faird N, Sinha V (2002) Fluoxetine pharmacokinetics in pediatric patients. J Clin Psychopharmacol 22:568–575
Yasukochi Y, Satta Y (2011) Evolution of the CYP2D gene cluster in humans and four non-human primates. Genes Genet Syst 86:109–116
Yu YW, Tsai SJ, Hong CJ, Chen TJ, Chen MC, Yang CW (2005) Association study of a monoamine oxidase A gene promoter polymorphism with major depressive disorder and antidepressant response. Neuropsychopharmacology 30:1719–1723
Acknowledgments
All procedures conformed to the Guide for the Care and Use of Laboratory Animals, 8th edition, issued by the US National Academy of Sciences. Fluoxetine and norfluoxetine assays were performed at the West Coast Metabolomics Center, an NIH Regional Resource Core, under the supervision of Mine Palazoglu. Erica Unger, Pennsylvania State University, performed the CSF analysis. Andrew Campbell, PhD, MPH, Andrew Campbell Consulting, performed the PK analysis. The authors appreciate the contribution of CNPRC Research Services personnel in conducting the dosing and blood sampling for the single-dose studies and Alicia Bulleri for the additional technical assistance. This study was supported by NIH grants R01HD065826 (Golub) and OD011107 (Lewin).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Fluoxetine data of individual subjects from the single-dose studies. The MAOA genotype is based on MAOA VNTR polymorphisms of 5, 6, or 7 repeats in the promoter region. 5/5 females, 5/- males, 6/6 females, and 6/- males are classified as hi-MAOA based on high transcription rate. 7/7 females and 7/- males are classified as low-MAOA based on low transcription rates for this polymorphism. 5-HTTLPR genotype is based on short (S) and long (L) forms of the serotonin transporter linked polymorphic region (5HTTLPR), with the S form resulting in lower transcription rates. Males or females are classified by allele as L/L, S/L or S/S (JPEG 259 kb)
Rights and permissions
About this article
Cite this article
Golub, M.S., Hogrefe, C.E. Fluoxetine: juvenile pharmacokinetics in a nonhuman primate model. Psychopharmacology 231, 4041–4047 (2014). https://doi.org/10.1007/s00213-014-3537-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3537-y