Abstract
Rationale
In order to improve upon the pharmacological properties of the neuroactive steroid ganaxolone, it was used as the starting point in the design of novel neurosteroids that replace the 17β-acetyl side chain with an isoxazole bioisostere.
Objectives
UCI-50027 (3-[3α-hydroxy-3β-methyl-5α-androstan-17β-yl]-5-(hydroxymethyl)isoxazole) was designed as an orally active neuroactive steroid specifically targeted at the gamma-aminobutyric acid(A) receptor (GABAAR).
Methods
UCI-50027 was tested in vitro in Xenopus oocytes expressing human GABAARs and in vivo as an anticonvulsant, for ataxic effects and for anxiolytic activity.
Results
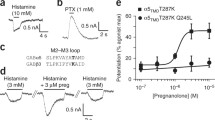
In vitro, UCI-50027 dose-dependently enhanced the activity of GABA at human α1β2γ2L, α2β1γ2L, and α4β3δ GABAARs. Consistent with its action as a positive allosteric modulator (PAM), it had no direct activity in the absence of GABA. UCI-50027 protected against acute pentylenetetrazol (PTZ)-induced convulsions with an ED50 of 6 mg/kg p.o. In the rotarod (RR) paradigm in mice, the AD50 (the ataxic dose where half of the animals fail the RR test) was found to be 38 mg/kg p.o., giving a therapeutic index (TI = RR AD50/PTZ ED50)∼6 versus 2.8 for ganaxolone. In the mouse-elevated plus maze (EPM) model for anxiety, UCI-50027 showed a minimum effective dose (MED) ≤0.3 mg/kg p.o. Thus, the TI (TI = RR AD50/EPM MED) for the compound as an anxiolytic is ≥127 versus 3.3 for ganaxolone.
Conclusions
UCI-50027 is an orally active neuroactive steroid with pharmacological activity consistent with a GABAAR PAM that has an improved separation between anticonvulsant/anxiolytic and rotarod effects, potent activity as an anticonvulsant and anxiolytic when compared to ganaxolone.




Similar content being viewed by others
Abbreviations
- ACN:
-
Acetonitrile
- AD50 :
-
Ataxogenic half-maximal dose where half of the mice fail the RR assay
- ANOVA:
-
Analysis of variance
- BZ:
-
Benzodiazepine
- CNS:
-
Central nervous system
- DMRM:
-
Daughter multiple reaction monitoring
- DMSO:
-
Dimethyl sulfoxide
- EC10 :
-
Concentration that evokes 10 % of the maximum response
- EC50 :
-
Concentration eliciting half the maximum response
- EC100 :
-
Concentration that evokes a maximum response
- ED50 :
-
Effective dose of drug at which half of the animals respond
- EPM:
-
Elevated plus maze
- GABAAR:
-
Gamma-aminobutyric acid(A) receptor
- Ganaxolone:
-
3α-hydroxy-3β-methyl-5α-pregnan-20-one
- HPβCD:
-
2-Hydroxypropyl-β-cyclodextrin
- HPLC:
-
High performance liquid chromatograph
- IACUC:
-
Institutional Animal Care and Use Committee
- LC/MS:
-
Liquid chromatography/mass spectrometry
- LGIC:
-
Ligand-gated ion channel
- MED:
-
Minimum effective dose
- MTBE:
-
Methyl tert-butyl ether
- PAM:
-
Positive allosteric modulator
- PD:
-
Pharmacodynamic
- PK:
-
Pharmacokinetic
- PTZ:
-
Pentylenetetrazol
- RR:
-
Rotarod
- SAR:
-
Structure-activity relationship
- SEM:
-
Standard error of the mean
- UCI-50027:
-
3-[3α-hydroxy-3β-methyl-5α-androstan-17β-yl]-5-(hydroxymethyl)isoxazole
- UCI-50031:
-
(S)-3-[3α-hydroxy-3β-methyl-5α-androstan-17β-yl]-5-(1-hydroxyethyl)isoxazole
References
Bialer M, Johannessen SI, Levy RH, Perucca E, Tomson T, White HS (2013) Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI). Epilepsy Res 103:2–30
Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW (1997) Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20 one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J Pharmacol Exp Ther 280:1284–1295
Gee KW, Tran MB, Hogenkamp DJ, Johnstone TB, Bagnera RE, Yoshimura RF, Huang J-C, Belluzzi JD, Whittemore ER (2010) Limiting activity at β1-subunit-containing GABAA receptor subtypes reduces ataxia. J Pharmacol Exp Ther 332:1040–1053
Hawkinson JE, Acosta-Burruel M, Yang KC, Hogenkamp DJ, Chen JS, Lan NC, Drewe JA, Whittemore ER, Woodward RM, Carter RB, Upasani RB (1998) Substituted 3β-phenylethynyl derivatives of 3α-hydroxy-5α-pregnan-20-one: remarkably potent neuroactive steroid modulators of γ-aminobutyric acidA receptors. J Pharmacol Exp Ther 287:198–207
Hogenkamp DJ (2013) Novel 17β-heteraryl-substituted steroids as modulators of GABAA receptors. International Published Application WO 2013/019711 A2, Feb. 7, 2013
Hogenkamp DJ, Tahir SH, Gee KW, Bolger MB et al (1997) Synthesis and in vitro activity of 3α-substituted-3β-hydroxypregnan-20-ones: allosteric modulators of the GABAA receptor. J Med Chem 40:61–72
Hogenkamp DJ, Johnstone TBC, Huang J-C, Li W-Y, Tran M, Whittemore ER, Bagnera RE, Gee KW (2007) Enaminone amides as novel orally active modulators of GABAA receptors. J Med Chem 50:3369–3379
Litchfield JT Jr, Wilcoxon FJ (1949) A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 96:99–113
Monaghan EP, McAuley JW, Data JL (1999) Ganaxolone: a novel positive allosteric modulator of the GABA(A) receptor complex for the treatment of epilepsy. Expert Opin Investig Drugs 8:1663–1671
Ng HJ, Whittemore ER, Tran MB, Hogenkamp DJ, Broide RS, Johnstone TB, Zheng L, Stevens KE, Gee KW (2007) Nootropic α7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proc Natl Acad Sci U S A 104:8059–8064
Nohria V, Tsai J, Shaw K, Rogawski MA, Pieribone VA, Farfel G (2010) Ganaxolone in progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X). Epilepsy Res 92:104–107
Ramu K, Lam GN, Chien B (2001) A high performance liquid chromatography-tandem mass spectrometric method for the determination of pharmacokinetics of ganaxolone in rat, monkey, dog and human plasma. J Chrom B 751:49–59
Reddy DS, Rogawski MA (2012) Neurosteroids-endogenous regulators of seizure susceptibility and role in the treatment of epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper’s basic mechanisms of the epilepsies, 4th edn. National Center for Biotechnology Information, Bethesda, pp 894–1002
Swerdlow M, Chakraborty SK, Zahangir MA (1971) A trial of CT1341. Br J Anaesth 43:1075–1080
Tsao CY (2009) Current trends in the treatment of infantile spasms. Neuropsychiatr Dis Treat 5:289–299
Yoshimura RF, Tran MB, Hogenkamp DJ, Johnstone TBJ, Gee KW (2013) Limited central side effects of a β-subunit subtype selective GABAA receptor allosteric modulator. J Psychopharmacol. doi:10.1177/0269881113507643
Acknowledgments
Grant was from the Center for Autism Research & Translation at the University of California Irvine to K.W.G.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hogenkamp, D.J., Tran, M.B., Yoshimura, R.F. et al. Pharmacological profile of a 17β-heteroaryl-substituted neuroactive steroid. Psychopharmacology 231, 3517–3524 (2014). https://doi.org/10.1007/s00213-014-3494-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3494-5