Abstract
Rationale
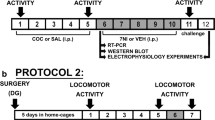
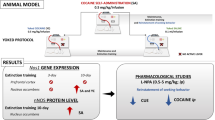
Repeated cocaine administration induces behavioral sensitization in about 50 % of treated animals. Nitric oxide could be involved in the acquisition and maintenance of behavioral cocaine effects, probably by activation of neuronal nitric oxide synthase (nNOS)/NO/soluble guanylyl cyclase (sGC)/cyclic guanosine monophosphate (cGMP) signaling pathway, since inhibition of the nNOS enzyme attenuates development of sensitization in rats. On the other hand, increased cGMP availability by phosphodiesterase 5 inhibitors has been correlated to the misuse and recreational use of these agents and also to the concomitant use with illicit drugs in humans. Hippocampus is an important brain region for conditioning to general context previously associated to drug availability, influencing drug-seeking behavior and sensitization. Moreover, cocaine and other drugs of abuse can affect the strength of glutamate synapses in this structure, lastly modifying neuronal activity in main regions of the reward circuitry.
Objective
The objective of this study is to determine whether the pharmacological manipulation of nNOS/NO/sGC/cGMP signaling pathway altered changes induced by repeated cocaine exposure.
Results
The present investigation showed a relationship between behavioral cocaine sensitization, reduced threshold to generate long-term potentiation (LTP) in hippocampal dentate gyrus, and increased nNOS activity in this structure. However, when nNOS or sGC were inhibited, the number of sensitized animals was reduced, and the threshold to generate LTP was increased. The opposite occurred when cGMP availability was increased.
Conclusion
We demonstrate a key role of the nNOS activity and NO/sGC/cGMP signaling pathway in the development of cocaine sensitization and in the associated enhancement of hippocampal synaptic transmission.




Similar content being viewed by others
References
Badanich KA, Adler KJ, Kirstein CL (2006) Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol 550:95–106
Bagetta G, Rodino P, Arabia A, Massoud R, Paoletti AM, Nistico R, Passantino L, Preziosi P (1999) Systemic administration of cocaine, given alone or in combination with sensory stimuli, differentially affects L-arginine-nitric oxide metabolism in discrete regions of the brain of rat. Neurosci Lett 266:153–156
Belujon P, Grace AA (2011) Hippocampus, amygdala and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci 1216:114–21
Bhargava HN, Kumar S (1997) Sensitization to the locomotor stimulant activity of cocaine is associated with increases in nitric oxide synthase activity in brain regions and spinal cord of mice. Pharmacology 55:292–298
Boccia MM, Blake MG, Krawczyk MC, Baratti CM (2011) Sildenafil, a selective phosphodiesterase type 5 inhibitor, enhances memory reconsolidation of an inhibitory avoidance task in mice. Behav Brain Res 220:319–324
Boudreau AC, Wolf ME (2005) Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci 25:9144–9151
Bredt DS, Hwang PM, Snyder SH (1990) Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347:768–770
Di CP, Cardinal RN, Cowell RA, Little SJ, Everitt BJ (2001) Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci 21:9471–9477
Everitt BJ, Wolf ME (2002) Psychomotor stimulant addiction: a neural systems perspective. J Neurosci 22:3312–3320
French SJ, Totterdell S (2002) Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol 446:151–165
Garthwaite J (2008) Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci 27:2783–2802
Garthwaite J (2010) New insight into the functioning of nitric oxide-receptive guanylyl cyclase: physiological and pharmacological implications. Mol Cell Biochem 334:221–232
Groenewegen HJ, Wright CI, Beijer AV, Voorn P (1999) Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci 877:49–63
Itzhak Y (1996) Attenuation of cocaine kindling by 7-nitroindazole, an inhibitor of brain nitric oxide synthase. Neuropharmacology 35:1065–1073
Itzhak Y (1997) Modulation of cocaine- and methamphetamine-induced behavioral sensitization by inhibition of brain nitric oxide synthase. J Pharmacol Exp Ther 282:521–527
Itzhak Y, Ali SF, Martin JL, Black MD, Huang PL (1998) Resistance of neuronal nitric oxide synthase-deficient mice to cocaine-induced locomotor sensitization. Psychopharmacology (Berl) 140:378–386
Itzhak Y, Roger-Sanchez C, Kelley JB, Anderson KL (2010) Discrimination between cocaine-associated context and cue in a modified conditioned place preference paradigm: role of the nNOS gene in cue conditioning. Int J Neuropsychopharmacol 13:171–180
Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology 146:373–90
Kelley AE (1999) Neural integrative activities of nucleus accumbes subregions in relation to learning and motivation. Psychobiology 27:198–213
Kelley AE (2004) Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev 27:765–776
Kelley AE, Domesick VB (1982) The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience 7:2321–2335
Kim HS, Park WK (1995) Nitric oxide mediation of cocaine-induced dopaminergic behaviors: ambulation-accelerating activity, reverse tolerance and conditioned place preference in mice. J Pharmacol Exp Ther 275:551–557
Knowles RG, Salter M (1998) Measurement of NOS activity by conversion of radiolabeled arginine to citrulline using ion-exchange separation. Methods Mol Biol 100:67–73
Kulkarni SK, Dhir A (2007) Possible involvement of L-arginine-nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) signaling pathway in the antidepressant activity of berberine chloride. Eur J Pharmacol 569:77–83
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23:649–711
McCambridge J, Mitcheson L, Hunt N, Winstock A (2006) The rise of Viagra among British illicit drug users: 5-year survey data. Drug Alcohol Rev 25:111–113
Mustafa AK, Gadalla MM, Snyder SH (2009) Signaling by gasotransmitters. Sci Signal 2:68
Nasif FJ, Hu XT, Ramirez OA, Perez MF (2011) Inhibition of neuronal nitric oxide synthase prevents alterations in medial prefrontal cortex excitability induced by repeated cocaine administration. Psychopharmacology (Berl) 218:323–330
Orsini C, Izzo E, Koob GF, Pulvirenti L (2002) Blockade of nitric oxide synthesis reduces responding for cocaine self-administration during extinction and reinstatement. Brain Res 925:133–140
Parent A (1990) Extrinsic connections of the basal ganglia. Trends Neurosci 13:254–8
Perez MF, Gabach LA, Almiron RS, Carlini VP, de Barioglio S, Ramirez OA (2010) Different chronic cocaine administration protocols induce changes on dentate gyrus plasticity and hippocampal dependent behavior. Synapse 64:742–753
Phillips RG, LeDoux JE (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106:274–285
Pierce RC, Bell K, Duffy P, Kalivas PW (1996) Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci 16:1550–1560
Prast H, Philippu A (2001) Nitric oxide as modulator of neuronal function. Prog Neurobiol 64:51–68
Puzzo D, Sapienza S, Arancio O, Palmeri A (2008) Role of phosphodiesterase 5 in synaptic plasticity and memory. Neuropsychiatr Dis Treat 4:371–387
Puzzo D, Staniszewski A, Deng SX, Privitera L, Leznik E, Liu S, Zhang H, Feng Y, Palmeri A, Landry DW, Arancio O (2009) Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer's disease mouse model. J Neurosci 29:8075–8086
Robbins TW, Ersche KD, Everitt BJ (2008) Drug addiction and the memory systems of the brain. Ann N Y Acad Sci 1141:1–21
Robinson TE, Berridge KC (2000) The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95(Suppl 2):S91–117
Rogerio R, Takahashi RN (1992) Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol Biochem Behav 43:631–633
Sammut S, West AR (2008) Acute cocaine administration increases NO efflux in the rat prefrontal cortex via a neuronal NOS-dependent mechanism. Synapse 62:710–713
Sammut S, Threlfell S, West A (2010) Nitric oxide-soluble guanylyl cyclase signaling regulates corticostriatal transmission and short-term synaptic plasticity of striatal projection neurons recorded in vivo. Neuropharmacology 58:624
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology 158:343–59
Smith KM, Romanelli F (2005) Recreational use and misuse of phosphodiesterase 5 inhibitors. J Am Pharm Assoc 45:63–72
Steketee JD (2005) Cortical mechanisms of cocaine sensitization. Crit Rev Neurobiol 17:69–86
Tahsili-Fahadan P, Yahyavi-Firouz-Abadi N, Orandi AH, Esmaeili B, Basseda Z, Dehpour AR (2006) Rewarding properties of sildenafil citrate in mice: role of the nitric oxide-cyclic GMP pathway. Psychopharmacology (Berl) 185:201–207
Teyler TJ, DiScenna P (1987) Long-term potentiation. Annu Rev Neurosci 10:131–161
Thompson AM, Gosnell BA, Wagner JJ (2002) Enhancement of long-term potentiation in the rat hippocampus following cocaine exposure. Neuropharmacology 42:1039–1042
Thompson AM, Swant J, Gosnell BA, Wagner JJ (2004) Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience 127:177–185
Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL (2001) Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 292:1175–1178
Wolf ME (2002) Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv 2:146–157
Yamaguchi M, Suzuki T, Seki T, Namba T, Liu J, Arai H, Hori T, Shiga T (2005) Decreased cell proliferation in the dentate gyrus of rats after repeated administration of cocaine. Synapse 58:63–71
Acknowledgment
We thank Kerstin Ford who assisted us with the manuscript edition.
Funding source
Funding for this study was provided by SECyT (PICT number 2476), CONICET (PIP number 6381), and MINCyT (PID 100). The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gabach, L.A., Carlini, V.P., Monti, M.C. et al. Involvement of nNOS/NO/sGC/cGMP signaling pathway in cocaine sensitization and in the associated hippocampal alterations: does phosphodiesterase 5 inhibition help to drug vulnerability?. Psychopharmacology 229, 41–50 (2013). https://doi.org/10.1007/s00213-013-3084-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3084-y