Abstract
Rationale
Increased appetite and weight gain after cessation is a deterrent for quitting smoking. Attempts to understand the mechanism for these effects using animals have been hampered by the difficulty or inconsistency of modeling the effects seen in humans.
Objective
To examine the effects of extended daily access to intravenous nicotine, via programmed infusions, on body weight and meal patterns in rats.
Methods
Intravenous (IV) nicotine infusions (0.06 mg/kg/inf) were administered noncontingently, every 30 min throughout the dark cycle and the last 3 h of the light cycle, to emulate self-administration. The effect of these infusions on food intake, meal patterns, and weight change were examined relative to a control group during treatment and in a post-nicotine phase.
Results
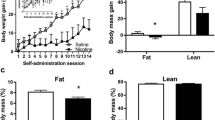
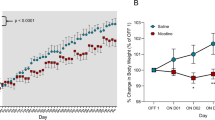
Nicotine-treated rats gained half the weight that vehicle treated animals gained and ate approximately 20 % less food overall than vehicle-treated rats. Whereas a compensatory increase in meal frequency occurred during the dark period to account for smaller meals, no compensation was observed throughout the light period. In a post-nicotine phase, the nicotine group maintained a lower weight for 1 week and then gained weight back to control levels. The rate of weight gain post-cessation was faster in animals that had received nicotine compared to controls.
Conclusion
Compared to previous studies examining the effects of minipump or intraperitoneal injections of nicotine on food intake, the present study was able to detect previously unknown circadian differences in meal patterns which will be important in the development of smoking cessation and weight gain prevention drugs.



Similar content being viewed by others
References
Bellinger L, Cepeda-Benito A, Bullard RL, Wellman PJ (2003a) Effect of i.c.v infusion of the α-MSH agonist MTII on meal patterns in male rats following nicotine withdrawal. Life Sci 73:1861–1872
Bellinger L, Cepeda-Benito A, Wellman PJ (2003b) Meal patterns in male rats during and after intermittent nicotine administration. Pharmacol Biochem Behav 74:495–504
Benowitz NL (1983) The use of biologic fluid samples in assessing tobacco smoke consumption. In: Grabowski J, Bell CS (ed) Measurement in the analysis and treatment of smoking behavior. NIDA research monograph no. 48. US Government Printing Office, Washington DC
Borrelli B, Spring B, Niaura R, Kristeller J, Ockene JK, Keuthen NJ (1999) Weight suppression and weight rebound in ex-smokers treated with fluoxetine. J Consult Clin Psychol 67(1):124–131
Bowen DJ, Spring B, Fox E (1991) Tryptophan and high-carbohydrate diets as adjuncts to smoking cessation therapy. J Behav Med 14(2):97–110
Buttner D, Wollnik F (1984) Strain-differentiated circadian and ultradian rhythms in locomotor activity of the laboratory rat. Behav Genet 14(2):137–152
Camp DE, Klesges RC, Relyea G (1993) The relationship between body weight concerns and adolescent smoking. Health Psychol 12(1):24–32
Chiolero A, Faeh D, Paccaud F, Cornuz J (2008) Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr 87(4):801–809
Dandekar MP, Nakhate KT, Kokare DM, Subhedar (2011) Effect of nicotine on feeding and body weight in rats. Involvement of cocaine- and amphetamine-regulated transcript peptide. Behav Brain Res 219:31–38
DiLeone RJ, Taylow JR, Picciotto MR (2012) The drive to eat: comparisons and distinctions between mechanisms of food reward and drug addiction. Nat Neurosci 15(10):1330–1335
Donny ED, Caggiula AR, Rose C, Jacobs KS, Mielke MM, Sved AF (2000) Differential effects of response-contingent and response-independent nicotine in rats. Eur J Pharmacol 402:231–240
Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF (2011) The reinforcement-enhancing effects of nicotine: implications for the relationship between smoking, eating, and weight. Physiol Behav 104:143–148
Fekete EM, Zhao Y, Szucs A, Sabino V, Cottone P, Rivier J, Vale WW, Koob GF, Zorrilla EP (2011) Systematic urocortin 2, but not urocortin 1 or stressin1-A suppresses feeing via CRF2 receptors without malaise and stress. Br J Pharmacol 164:1959–1975
Flegal KM, Troiano RP, Pamuk ER, Kuczmarski RJ, Campbell SM (1995) The influence of smoking cessation on the prevalence of overweight in the united states. N Engl J Med 333:1165–1170
Flegal KM, Carroll MD, Ogden CI, Curtin LR (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303(3):235–241
Grunberg NE, Bowen DJ (1985) The role of physical activity in nicotine’s effects on body weight. Pharmacol Biochem Behav 23:851–854
Grunberg NE, Bowen DJ, Maycock VA, Nespor SM (1985) The importance of sweet taste and caloric content in the effects of nicotine on specific food consumption. Psychopharmacology 87:198–203
Grunberg NE, Bowen DJ, Winders SE (1986) Effects of nicotine on body weight and food consumption in female rats. Psychopharmacology 90:101–105
Guan G, Kramer SF, Bellinger LL, Wellman PJ, Kramer PR (2004) Intermittent nicotine administration modulates food intake in rats by acting on nicotine receptors localized to the brainstem. Life Sci 74:2725–2737
Hanson HM, Ivester CA, Morton BR (1979) Nicotine self-administration in rats. In: Krasnegor NA(ed) Cigarette smoking as a dependence process, NIDA research monograph no. 23. Public Health Service, U.S. Department of Heath Education and Welfare, Washington DC
Henningfield JE, Keenan RM (1993) Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol 61(5):742–750
Henningfield JE, Stapleton JM, Benowitx NL, Grayson RF, London ED (1993) Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend 33:23–29
Jeffery RW, Hennrikus DJ, Lando HA, Murray DM, Liu JW (2000) Reconciling conflicting findings regarding postcessation weight concerns and success in smoking cessation. Health Psychol 19(3):242–246
Jo YH, Talmage DA, Role LW (2002) Nicotinic receptor-mediated effects on appetite and food intake. J Neurobiol 53(4):618–632
Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD (2000) Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinol 141:3623–3629
Keenan KP, How CM, Mixson L, McCoy CL, Coleman JB, Mattson BA, Ballam GA, Grumprecht LA, Soper KA (2005) Diabesity: a polygenic model of dietary-induced obesity from ad donnylibitum overfeeding of Sprague–Dawley rats and its modulation by moderate and marked dietary restriction. Toxicol Pathol 33(6):650–674
Klesges RC, Meyers AW, Klesges LM, LaVasque ME (1989) Smoking, body weight, and their effect on smoking behavior: a comprehensive review of the literature. Psychol Bull 106(2):204–230
Koob GF, Volkow ND (2009) Neurocircuitry of addiction. Neuropsychopharmacology 35(1):217–238
Kramer PR, Kramer SF, Marr K, Guan G, Wellman PJ, Bellinger LL (2007) Nicotone administration effects on feeing cocaine–amphetamine regulated transcript (CART) expression in the hypothalamus. Regul Pept 138:66–71
Le Magnen J (1985) Hunger. Cambridge University Press, Cambridge
Leibowitz SF, Alexander JT (1991) Analysis of neuropeptide Y-induced feeding: dissociation of Y1 and Y2 receptor effects on natural meal patterns. Peptides 12:1251–1260
Li MD, Kane JK, Parker SL, McAllen K, Matta SG, Sharp BM (2000) Nicotine administration enhances NPY expression in the rat hypothalamus. Brain Res 867:157–164
Mangubat M, Lufty K, Lee ML, Pulido L, Stout D, Davis R, Shin C, Shahbazian M, Seasholtz S, Sinha-Hikim A, Sinha-Hikim I, O’Dell LE, Lyzlov A, Liu Y, Friedman TC (2012) Effect of nicotine on body composition in mice. J Endocrinol 212:317–326
Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F (2000) Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition 16:843–857
Meister B, Gomuc B, Suarez E, Ishii Y, Durr K, Gillberg L (2006) Hypothalamic proopiomelanocortin (POMC) a cholinergic phenotype. Eur J Neurosci 24:2731–2740
Miyata G, Meguid MM, Fetissov SO, Torelli GF, Kim H (1999) Nicotine’s effect on hypothalamic neurotransmitters and appetite regulation. Surg 126:255–263
Miyata G, Meguid MM, Varma M, Fetissov SO, Kim H (2001) Nicotine alters the usual reciprocity between meal size and meal number in female rat. Physiol Behav 74:169–176
O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF (2007) Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther 320:180–193
Paterson NE, Markou A (2004) Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology 173:64–72
Pisinger C, Jorgensen T (2007) Waist circumference and weight following smoking cessation in the general population: the Inter99 study. Prev Med 44:290–295
Pistelli F, Aquilini F, Carrozzi L (2009) Weight gain after smoking cessation. Monaldi Arch Chest Dis 71(2):81–87
Rodin J (1987) Weight change following smoking cessation: the role of food intake and exercise. Addict Behav 12:303–317
Rowland NE, Robertson K, Soti F, Kem WR (2008) Nicotine analog inhibition of nicotine self-administration. Psychopharmacology 199:605–613
Salin-Pascual RJ, Moro-Lopez ML, Gonzalez-Sanchez, Blanco-Centurion C (1999) Changes in sleep after acute and repeated administration of nicotine in the rat. Psychopharmacology 145:133–138
Sanderson EM, Drasdo AL, McCrea K, Wonnacott S (1993) Upregulation of nicotinic receptors following continuous infusion of nicotine is brain-region-specific. Brain Res 617:349–352
Saules KK, Pomerleau CS, Snedecor SM, Brouwer RN, Rosenberg EE (2004) Effects of disordered eating and obesity on weight, craving, and food intake during ad libitum smoking and abstinence. Eat Behav 5:353–363
Spring B, Pagoto S, McChargue D, Hedeker D, Werth J (2003) Altered reward value of carbohydrate snacks for female smokers withdrawn from nicotine. Pharmacol Biochem Behav 76:351–360
Swan GE, Carmelli D (1995) Characteristics associated with excessive weight gain after smoking cessation in men. Am J Public Health 85:73–77
Wellman PJ, Bellinger LL, Cepeda-Benito A, Susabda A, Ho DH, Davis KW (2005) Meal patterns and body weight after nicotine in male rats as a function of chow or high-fat diet. Pharmacol Biochem Behav 82:627–634
Zoli M, Picciotto MR (2012) Nicotinic regulation of energy homeostasis. Nicotine Tob Res 14(11):1270–1290
Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF (2005) Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol 288:R1450–R1467
Acknowledgment
N.E. Rowland was supported by NIH DA019288.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material

Fig. S1
Total meal size. Shown are mean +/- SEM total number of pellets per meal, averaged over the ranges of days indicated during 23 hours (a), the dark period (b), or the light period (c), in animals that received either vehicle (n = 9) or .06 mg/kg/inf nicotine (n = 8) during the treatment phase. ^P < .01, *P < 0.05, **P < 0.01 difference between vehicle and nicotine (JPEG 59 kb)
High resolution image
(TIFF 1061 kb)

Fig. S2
Total meal number. Shown are mean +/- SEM number of meals, averaged over the ranges of days indicated during 23 hours (a), the dark period (b), or the light period (c), in animals that received either vehicle (n = 9) or .06 mg/kg/inf nicotine (n = 8) during the treatment phase. ^P < .01, *P < 0.05, **P < 0.01 difference between vehicle and nicotine (JPEG 63 kb)
High resolution image
(TIFF 1088 kb)
Table S1
F values for the changes in body weight over each day of the baseline, treatment, and cessation phases between the control and the .06 mg/kg inf nicotine groups (DOCX 18.6 kb)
Table S2
F values for all meal pattern variables of interest, with days clustered into groups, throughout the treatment phase for the control and nicotine groups (DOCX 23 kb)
Table S3
F values for all meal pattern variables of interest, with days clustered into groups, throughout the cessation phase for the control and nicotine groups (DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Grebenstein, P.E., Thompson, I.E. & Rowland, N.E. The effects of extended intravenous nicotine administration on body weight and meal patterns in male Sprague–Dawley Rats. Psychopharmacology 228, 359–366 (2013). https://doi.org/10.1007/s00213-013-3043-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3043-7