Abstract
Rationale
Existing animal models of impulsivity frequently use food restriction to increase subjects’ motivation. In addition, behavioral tasks that assess impulsive choice typically involve the use of reinforcers with dissimilar caloric content. These factors represent energy-homeostasis limitations, which may confound the interpretation of results and limit the applicability of these models.
Objectives
This study was aimed at validating face and convergent validities of a modified adjusting delay task, which assesses impulsive choice between isocaloric reinforcers in ad libitum fed rats.
Methods
Male Wistar rats (n = 18) were used to assess the preferredness and reinforcing efficacy of a “supersaccharin” solution (1.5% glucose/0.4% saccharin) over a 1.5% glucose solution. A separate group of rats (n = 24) was trained in a modified adjusting delay task, which involved repeated choice between the glucose solution delivered immediately and the supersaccharin solution delivered after a variable delay. To pharmacologically validate the task, the effects of the 5-HT2A/C receptor agonist (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(±)-DOI] and the 5-HT1A receptor agonist (±)-8-hydroxy-2-(dipropylamino)tetralin hydrobromide [(±)-8-OH-DPAT] on impulsive choice were then evaluated.
Results
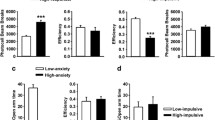
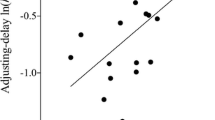
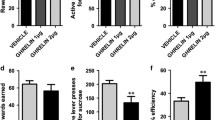
Supersaccharin was highly reinforcing and uniformly preferred over the glucose solution by all subjects. Rats quickly learned the task, and impulsivity was a very stable and consistent trait. DOI and 8-OH-DPAT significantly and dose dependently increased impulsive choice in this modified adjusting delay task.
Conclusions
We validated a rodent task of impulsive choice, which eliminates typical energy-homeostasis limitations and, therefore, opens new avenues in the study of impulsivity in preclinical feeding and obesity research.




Similar content being viewed by others
References
Ahlenius S, Hillegaart V, Wijkstrom A (1989) Evidence for selective inhibition of limbic forebrain dopamine synthesis by 8-OH-DPAT in the rat. Naunyn Schmiedebergs Arch Pharmacol 339:551–556
Ainslie G (1975) Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull 82:463–496
Arborelius L, Chergui K, Murase S, Nomikos GG, Hook BB, Chouvet G, Hacksell U, Svensson TH (1993) The 5-HT1A receptor selective ligands, (R)-8-OH-DPAT and (S)-UH-301, differentially affect the activity of midbrain dopamine neurons. Naunyn Schmiedebergs Arch Pharmacol 347:353–362
Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL (1993) Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem 268:23422–23426
Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320:1352–1355
Beshel J, Kopell N, Kay LM (2007) Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci 27:8358–8365
Bi S, Robinson BM, Moran TH (2003) Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol 285:R1030–R1036
Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ (2001) Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292:2499–2501
Carli M, Samanin R (2000) The 5-HT(1A) receptor agonist 8-OH-DPAT reduces rats’ accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: role of presynaptic 5-HT(1A) receptors. Psychopharmacology (Berl) 149:259–268
Carr KD, Kim G, Cabeza de Vaca S (2000) Hypoinsulinemia may mediate the lowering of self-stimulation thresholds by food restriction and streptozotocin-induced diabetes. Brain Res 863:160–168
Carr KD, Tsimberg Y, Berman Y, Yamamoto N (2003) Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience 119:1157–1167
Cassin SE, von Ranson KM (2005) Personality and eating disorders: a decade in review. Clin Psychol Rev 25:895–916
Chamberlain SR, Sahakian BJ (2007) The neuropsychiatry of impulsivity. Curr Opin Psychiatry 20:255–261
Chen NH, Reith ME (1995) Monoamine interactions measured by microdialysis in the ventral tegmental area of rats treated systemically with (+/−)-8-hydroxy-2-(di-n-propylamino)tetralin. J Neurochem 64:1585–1597
Cheng CM, Hicks K, Wang J, Eagles DA, Bondy CA (2004) Caloric restriction augments brain glutamic acid decarboxylase-65 and -67 expression. J Neurosci Res 77:270–276
Cottone P, Sabino V, Steardo L, Zorrilla EP (2007) FG 7142 specifically reduces meal size and the rate and regularity of sustained feeding in female rats: evidence that benzodiazepine inverse agonists reduce food palatability. Neuropsychopharmacology 32:1069–1081
Cottone P, Sabino V, Steardo L, Zorrilla EP (2008) Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology 33:524–535
Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP (2009a) CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci USA 106:20016–20020
Cottone P, Sabino V, Steardo L, Zorrilla EP (2009b) Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology 34:38–49
Crews FT, Boettiger CA (2009) Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav 93:237–247
Dalley JW, Mar AC, Economidou D, Robbins TW (2008) Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav 90:250–260
Davis C (2009) Psychobiological traits in the risk profile for overeating and weight gain. Int J Obes (Lond) 33(Suppl 2):S49–S53
Davis C, Levitan RD, Carter J, Kaplan AS, Reid C, Curtis C, Patte K, Kennedy JL (2008) Personality and eating behaviors: a case-control study of binge eating disorder. Int J Eat Disord 41:243–250
De Vry J, Schohe-Loop R, Heine HG, Greuel JM, Mauler F, Schmidt B, Sommermeyer H, Glaser T (1998) Characterization of the aminomethylchroman derivative BAY x 3702 as a highly potent 5-hydroxytryptamine1A receptor agonist. J Pharmacol Exp Ther 284:1082–1094
Di Marzo V, Matias I (2005) Endocannabinoid control of food intake and energy balance. Nat Neurosci 8:585–589
Eagle DM, Robbins TW (2003a) Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci 117:1302–1317
Eagle DM, Robbins TW (2003b) Lesions of the medial prefrontal cortex or nucleus accumbens core do not impair inhibitory control in rats performing a stop-signal reaction time task. Behav Brain Res 146:131–144
Evenden JL (1998a) The pharmacology of impulsive behaviour in rats II: the effects of amphetamine, haloperidol, imipramine, chlordiazepoxide and other drugs on fixed consecutive number schedules (FCN 8 and FCN 32). Psychopharmacology (Berl) 138:283–294
Evenden JL (1998b) The pharmacology of impulsive behaviour in rats IV: the effects of selective serotonergic agents on a paced fixed consecutive number schedule. Psychopharmacology (Berl) 140:319–330
Evenden JL (1999) Varieties of impulsivity. Psychopharmacology (Berl) 146:348–361
Evenden JL, Ryan CN (1999) The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 146:413–421
Fulton S, Woodside B, Shizgal P (2000) Modulation of brain reward circuitry by leptin. Science 287:125–128
Glennon RA, Titeler M, Lyon RA, Slusher RM (1988) N, N-di-n-propylserotonin: binding at serotonin binding sites and a comparison with 8-hydroxy-2-(di-n-propylamino)tetralin. J Med Chem 31:867–870
Haleem DJ, Haider S (1996) Food restriction decreases serotonin and its synthesis rate in the hypothalamus. Neuroreport 7:1153–1156
Harkness JEWJ (1989) The biology and medicine of rabbits and rodents, 3rd edition. Lea and Febiger, Philadelphia
Helton DR, Colbert WE (1994) Alterations of in-vitro 5-HT receptor pharmacology as a function of multiple treatment with 5-hydroxytryptamine or 8-hydroxy-2-(di-N-propylamino) tetralin in rat isolated aorta, uterus and fundus, and guinea-pig isolated trachea. J Pharm Pharmacol 46:902–905
Hillegaart V, Hjorth S (1989) Median raphe, but not dorsal raphe, application of the 5-HT1A agonist 8-OH-DPAT stimulates rat motor activity. Eur J Pharmacol 160:303–307
Ichikawa J, Kuroki T, Kitchen MT, Meltzer HY (1995) R(+)-8-OH-DPAT, a 5-HT1A receptor agonist, inhibits amphetamine-induced dopamine release in rat striatum and nucleus accumbens. Eur J Pharmacol 287:179–184
Johansson A, Fredriksson R, Winnergren S, Hulting AL, Schioth HB, Lindblom J (2008) The relative impact of chronic food restriction and acute food deprivation on plasma hormone levels and hypothalamic neuropeptide expression. Peptides 29:1588–1595
Knott PJ, Curzon G (1974) Effect of increased rat brain tryptophan on 5-hydroxytryptamine and 5-hydroxyindolyl acetic acid in the hypothalamus and other brain regions. J Neurochem 22:1065–1071
Koskinen T, Ruotsalainen S, Puumala T, Lappalainen R, Koivisto E, Mannisto PT, Sirvio J (2000) Activation of 5-HT2A receptors impairs response control of rats in a five-choice serial reaction time task. Neuropharmacology 39:471–481
Koskinen T, Haapalinna A, Sirvio J (2003) Alpha-adrenoceptor-mediated modulation of 5-HT2 receptor agonist induced impulsive responding in a 5-choice serial reaction time task. Pharmacol Toxicol 92:214–225
Lindblom J, Johansson A, Holmgren A, Grandin E, Nedergard C, Fredriksson R, Schioth HB (2006) Increased mRNA levels of tyrosine hydroxylase and dopamine transporter in the VTA of male rats after chronic food restriction. Eur J Neurosci 23:180–186
Mar AC, Walker AL, Theobald DE, Eagle DM, Robbins TW (2011) Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci 31:6398–6404
Markou MAGaA (2000) Animal models of psychiatric disorders. Neuropsychopharmacology: the fifth generation of progress. LWW, Philadelphia
Mazur JE (1986) Fixed and variable ratios and delays: further tests of an equivalence rule. J Exp Psychol Anim Behav Process 12:116–124
Mazur JE (1988) Estimation of indifference points with an adjusting-delay procedure. J Exp Anal Behav 49:37–47
Nelson DL, Lucaites VL, Wainscott DB, Glennon RA (1999) Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, -HT(2B) and 5-HT2C receptors. Naunyn Schmiedebergs Arch Pharmacol 359:1–6
O’Donnell JM, Seiden LS (1982) Effects of monoamine oxidase inhibitors on performance during differential reinforcement of low response rate. Psychopharmacology (Berl) 78:214–218
O’Donnell JM, Seiden LS (1984) Altered effects of desipramine on operant performance after 6-hydroxydopamine-induced depletion of brain dopamine or norepinephrine. J Pharmacol Exp Ther 229:629–635
Pan Y, Berman Y, Haberny S, Meller E, Carr KD (2006) Synthesis, protein levels, activity, and phosphorylation state of tyrosine hydroxylase in mesoaccumbens and nigrostriatal dopamine pathways of chronically food-restricted rats. Brain Res 1122:135–142
Perry JL, Larson EB, German JP, Madden GJ, Carroll ME (2005) Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 178:193–201
Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME (2007) Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav 86:822–837
Potenza MN (2007) To do or not to do? The complexities of addiction, motivation, self-control, and impulsivity. Am J Psychiatry 164:4–6
Puder JJ, Munsch S (2010) Psychological correlates of childhood obesity. Int J Obes (Lond) 34(Suppl 2):S37–S43
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163:362–380
Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH (2010) Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry 67:831–839
Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC (1993) Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA 90:8547–8551
Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP (2006) Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 189:175–186
Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L Jr, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP (2009) The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology 34:1482–1493
Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP (2011) Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology 36:1207–1218
Schoenbaum G, Chiba AA, Gallagher M (1998) Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci 1:155–159
Schoenbaum G, Chiba AA, Gallagher M (2000) Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci 20:5179–5189
Schoffelmeer AN, Drukarch B, De Vries TJ, Hogenboom F, Schetters D, Pattij T (2011) Insulin modulates cocaine-sensitive monoamine transporter function and impulsive behavior. J Neurosci 31:1284–1291
Shrout PE, Fleiss JL (1979) Intraclass correlations: Uses in assessing rater reliability. Psychol Bull 86:420–428
Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL (2011) Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 180:129–137
Stanis JJ, Burns RM, Sherrill LK, Gulley JM (2008) Disparate cocaine-induced locomotion as a predictor of choice behavior in rats trained in a delay-discounting task. Drug Alcohol Depend 98:54–62
Torregrossa MM, Quinn JJ, Taylor JR (2008) Impulsivity, compulsivity, and habit: the role of orbitofrontal cortex revisited. Biol Psychiatry 63:253–255
Tricklebank MD, Forler C, Fozard JR (1984) The involvement of subtypes of the 5-HT1 receptor and of catecholaminergic systems in the behavioural response to 8-hydroxy-2-(di-n-propylamino)tetralin in the rat. Eur J Pharmacol 106:271–282
Valenstein ES, Cox VC, Kakolewski JW (1967) Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science 157:552–554
Waxman SE (2009) A systematic review of impulsivity in eating disorders. Eur Eat Disord Rev 17:408–425
Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW (2003) Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 167:304–314
Winstanley CA, Theobald DE, Dalley JW, Robbins TW (2005) Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology 30:669–682
Winstanley CA, Eagle DM, Robbins TW (2006) Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev 26:379–395
Wolinsky TD, Carr KD, Hiller JM, Simon EJ (1996) Chronic food restriction alters mu and kappa opioid receptor binding in the parabrachial nucleus of the rat: a quantitative autoradiographic study. Brain Res 706:333–336
Acknowledgments
We thank Frank Gibbs for technical assistance and Jina Kwak for the technical and editorial assistance. This publication was made possible by grant numbers DA023680, DA030425, MH091945, and AA016731 from the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH) and the National Institute on Alcohol Abuse and Alcoholism (NIAAA), and by the Peter Paul Career Development Professorship. The authors declare no conflict of interest. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Blasio, A., Narayan, A.R., Kaminski, B.J. et al. A modified adjusting delay task to assess impulsive choice between isocaloric reinforcers in non-deprived male rats: effects of 5-HT2A/C and 5-HT1A receptor agonists. Psychopharmacology 219, 377–386 (2012). https://doi.org/10.1007/s00213-011-2517-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2517-8