Abstract
Rationale
Experimental evidence indicates that nicotine causes long-lasting changes in the brain associated with behavior. Although much has been learned about factors participating in this process, less is known concerning the mechanisms and brain areas involved in nicotine preference.
Objectives
The objective of this study is to examine the participation of brain structures during the development of nicotine-conditioned place preference (CPP).
Methods
To identify brain regions activated in CPP, we have measured the levels of phosphorylated cyclic AMP response element binding protein (pCREB) and Fos protein using a behavioral CPP and conditioned place aversion (CPA) paradigms.
Results
Rats developed reliable and robust CPP and also CPA. During nicotine preference and reinstatement behaviors, a significant increase of both pCREB and Fos protein expression occurs in the nucleus accumbens (NAc) and ventral tegmental area (VTA) and also in the prefrontal cortex (PFC), dorsal striatum (DStr), amygdala, and hippocampus. These increases were abolished by the administration of mecamylamine or by a CPA protocol, showing a specific activation of pCREB in drug preference animals, mediated by nicotinic receptors. Specifically in the VTA, nicotine-induced preference and reinstatement of the preference caused the activation of dopaminergic and GABAergic cells in different proportions.
Conclusion
The results indicate that the phosphorylation of CREB and expression of Fos protein, as indicators of neural activity, accompany the acquisition and maintenance of nicotine-induced CPP but not CPA in mesolimbic areas (NAc, VTA, PFC, and DStr) as well as in memory consolidation structures (hippocampus and amygdala) and nicotinic receptor are involved in this process. Taken together, these studies identify the brain regions where pCREB activity is essential for nicotine preference.










Similar content being viewed by others
References
Berke JD, Hyman SE (2000) Addiction, dopamine and the molecular mechanisms of memory. Neuron 25:515–532
Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH (1997) Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci U S A 94:7041–7046
Brunzell DH, Russell DS, Picciotto MR (2003) In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem 84:1431–1441
Brunzell DH, Mineur YS, Neve RL, Picciotto MR (2009) Nucleus accumbens CREB activity is necessary for nicotine conditioned place preference. Neuropsychopharmacology 34(8):1993–2001
Calcagnetti DJ, Schechter MD (1994) Nicotine place preference using the biased method of conditioning. Prog Neuropsychopharmacol Biol Psychiatry 18:925–933
Carlezon WA Jr, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28:436–445
Cassel S, Carouge D, Gensburger C, Anglard P, Burgun C, Dietrich JB, Aunis D, Zwiller J (2006) Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol 70:487–492
Cogesshal RE, Lekan HA (1996) Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol 364:6–15
Dani JA, Heinemann S (1996) Molecular and cellular aspects of nicotine abuse. Neuron 16:905–908
Fisher JL, Pidoplichko VI, Dani JA (1998) Nicotine modifies the activity of ventral tegmental area dopaminergic neurons and hippocampal GABAergic neurons. J Physiol Paris 92:209–213
Forget B, Hamon M, Thiébot MH (2005) Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology 181:722–734
Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA (2000) Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther 294:1112–1119
Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B et al (1988) The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Acta Pathol Microbiol Immunol Scand 96:857–881
Hyman SE (2005) Addiction: a disease of learning and memory. Am J Psychiatry 162:1414–1422
Hyman SE, Malenka RC (2001) Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2:695–703
Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598
Kauer JA, Malenka RC (2007) Synaptic plasticity and addiction. Nat Rev Neurosci 8:844–858
Kelley AE (2004) Memory and addiction: review shared neural circuitry and molecular mechanisms. Neuron 44:161–179
Koob G, Kreek MJ (2007) Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164:1149–1159
Korzus E, Rosenfeld MG, Mayford M (2004) CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42:961–972
Laviolette SR, van der Kooy D (2003) Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol Psychiatry 8:50–59
Laviolette SR, Van der Kooy D (2004) The neurobiology of nicotine addiction: bridging the gap from molecules to behavior. Nat Rev Neurosci 5:55–65
Le Foll B, Goldberg SR (2005) Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 178:481–492
Le Foll B, Goldberg SR (2006) Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology 184:367–381
Liu ZH, Jin WQ (2004) Decrease of ventral tegmental area dopamine neuronal activity in nicotine withdrawal rats. NeuroReport 15:1479–1481
Mansvelder HD, McGehee DS (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27:349–357
Mansvelder HD, Keath JR, McGehee DS (2002) Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33:905–919
Miller CA, Marshall JF (2005) Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 47:873–884
Montag-Sallaz M, Welzl H, Kuhl D, Montag D, Schachner M (1999) Novelty-induced increased expression of immediate-early genes c-fos and arg 3.1 in the mouse brain. J Neurobiol 38:234–246
Nestler EJ (2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2:119–128
Nestler EJ (2002) Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem 78:637–647
Pandey SC, Roy A, Xu T, Mittal N (2001) Effects of protracted nicotine exposure and withdrawal on the expression and phosphorylation of the CREB gene transcription factor in rat brain. J Neurochem 77:943–952
Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177
Pidoplichko VI, DeBiasi M, Williams JT, Dani JA (1997) Nicotine activates and desensitizes midbrain dopamine neurons. Nature 390:401–404
Pluzarev O, Pandey SC (2004) Modulation of CREB expression and phosphorylation in the rat nucleus accumbens during nicotine exposure and withdrawal. J Neurosci Res 77:884–891
Robbins TW, Everitt BJ (1999) Drug addiction: bad habits add up. Nature 398:567–570
Robbins TW, Everitt BJ (2002) Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem 78:625–636
Salminen O, Lahtinen S, Ahtee L (1996) Expression of Fos protein in various rat brain areas following acute nicotine and diazepam. Pharmacol Biochem Behav 54:241–248
Salminen O, Seppä T, Gäddnäs H, Ahtee L (1999) The effects of acute nicotine on the metabolism of dopamine and the expression of Fos protein in striatal and limbic brain areas of rats during chronic nicotine infusion and its withdrawal. J Neurosci 19:8145–8151
Schiltz CA, Kelley AE, Landry CF (2005) Contextual cues associated with nicotine administration increase arc mRNA expression in corticolimbic areas of the rat brain. Eur J NeuroSci 21:1703–1711
Shaywitz AJ, Greenberg ME (1999) CREB: a stimulus-induced transcription factoractivated by diverse array of extracellular signals. Annu Rev Biochem 68:821–861
Stolerman IP, Jarvis MJ (1995) The scientific case that nicotine is addictive. Psychopharmacology 117:2–10
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462
Ungless MA, Whistler JL, Malenka RC, Bonci A (2001) Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411:583–587
Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP (2002) Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav 77:107–114
Walters CL, Cleck JN, Kuo YC, Blendy JA (2005) Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron 46:933–943
Wilmouth CE, Spear LP (2006) Withdrawal from chronic nicotine in adolescent and adult rats. Pharmacol Biochem Behav 85:648–657
Wolf ME (2003) LTP may trigger addiction. Mol Interv 3:248–252
Wu H, Zhou Y, Xiong ZQ (2007) Transducer of regulated CREB and late phase long-term synaptic potentiation. FEBS J 274:3218–3223
Acknowledgements
This work was supported in part by grants from Agencia Nacional de Promocion Cientifica y Tecnologica and CONICET (R.B.), Argentina. We thank M. P. Faillace and J. Zwiller for the helpful comments and discussion and Maria Rosato Siri for some help in the first version of the manuscript.
Competing interest statement
The authors declare that they have no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 1S
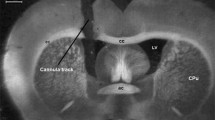
a Low magnification pictures shows the brain areas analyzed for VTA and NAc. NAcc NAc core, NAcs NAc shell. b The stringent criterion applied to pCREB and Fos quantification. a The picture shows pCREB-positive nuclei and a line used as a reference to measure the intensity of each nucleus, named 1, 2, 3, and 4. Scale bar 20 µm. b The y-axis represents intensity. The x-axis represents the distance of the line drew in a. Each number represents the four nucleus of figure a. Only the intensity values that were higher than a determined intensity (horizontal arrow) were considered positive (GIF 47.3 kb)
Rights and permissions
About this article
Cite this article
Pascual, M.M., Pastor, V. & Bernabeu, R.O. Nicotine-conditioned place preference induced CREB phosphorylation and Fos expression in the adult rat brain. Psychopharmacology 207, 57–71 (2009). https://doi.org/10.1007/s00213-009-1630-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1630-4