Abstract
Rationale
Relapse to drug use after periods of forced or self-imposed abstinence is a central problem in the treatment of addiction; therefore, identification of factors modulating the risk to relapse is a relevant goal of preclinical research.
Objectives
These experiments evaluated the influence of the amount of drug experienced, the duration of drug withdrawal, and individual liability on the propensity to cocaine-induced reinstatement of conditioned place preference (CPP).
Materials and methods
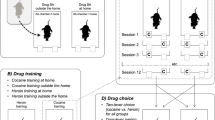
Mice from the inbred strains C57BL/6J and DBA/2J were trained for CPP with a high (20 mg/kg) or low (5 mg/kg) effective dose of cocaine. After CPP testing, all groups underwent extinction. Twenty-four hours after the extinction test, mice were challenged with saline, a cocaine dose unable to induce CPP (2.5 mg/kg) or an intermediate effective dose (10 mg/kg), and tested for CPP reinstatement. Additional groups of mice trained with the low cocaine dose were left undisturbed for 8 days after extinction test (long withdrawal), retested for extinction, and evaluated for prime-induced reinstatement (0, 2.5, 10 mg/kg of cocaine).
Results
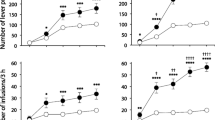
Mice trained with the high cocaine dose, but not with the low one, showed prime-induced reinstatement 24 h after the extinction test; DBA/2J mice trained with the low dose showed reinstatement after long withdrawal.
Conclusions
These results indicate that reinstatement of CPP by cocaine prime depends on the amount of drug experienced and on an interaction between individual liability and duration of drug abstinence and suggest that the risk to relapse into drug seeking is not prevented by moderated drug consumption.






Similar content being viewed by others
References
Badiani A, Cabib S, Puglisi-Allegra S (1992) Chronic stress induces strain-dependent sensitization to the behavioral effects of amphetamine in the mouse. Pharmacol Biochem Behav 43:53–60
Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL (2001) Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 155:18–26
Cabib S, Puglisi-Allegra S, Genua C, Simon H, Le Moal M, Piazza PV (1996) Dose-dependent aversive and rewarding effects of amphetamine as revealed by a new place preference conditioning apparatus. Psychopharmacol 125:92–96
Cabib S, Giardino L, Calzà L, Zanni M, Mele A, Puglisi-Allegra S (1998) Stress promotes major changes in dopamine receptor densities within the mesoaccumens and nigrostriatal systems. Neurosci 84:193–200
Cabib S, Orsini C, Le Moal M, Piazza PV (2000) Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science 289:463–465
D’Este L, Casini A, Puglisi-Allegra S, Cabib S, Renda TG (2007) Comparative immunohistochemical study of the dopaminergic systems in the two inbred mouse strains (C57BL/6J and DBA/2J). J Chem Neuroanat 33:67–74
De Jong IEM, Oitzl MS, de Kloet R (2007) Adrenalectomy prevents behavioral sensitization of mice to cocaine in a genotype-dependent manner. Behav Brain Res 177:329–339
Deroche V, Le Moal M, Piazza PV (1999) Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci 11:2731–2736
Deroche-Gamonet V, Martinez A, Le Moal M, Piazza PV (2003) Relationships between individual sensitivity to CS- and cocaine-induced reinstatement in the rat. Psychopharmacol 168:201–207
Everitt B, Wolf M (2002) Psychomotor stimulant addiction: a neural systems perspective. J Neurosci 22:3312–3320
George SR, Fan T, Ng GY, Jung SY, O’Dowd BF, Naranjo CA (1995) Low endogenous dopamine function in brain predisposes to high alcohol preference and consumption: reversal by increasing synaptic dopamine. J Pharmacol Exp Ther 273:373–379
Homberg JR, Raasø HS, Schoffelmeer ANM, de Vries TJ (2004) Individual differences in sensitivity to factors provoking reinstatement of cocaine-seeking behavior. Behav Brain Res 152:157–161
Itzhak Y, Martin JL (2002) Cocaine-induced place preference in mice: induction, extinction and reinstatement by related psychostimulants. Neuropsychopharmacol 26:130–134
Kruzich PJ, Jinlei X (2006) different patterns of pharmacological reinstatement of cocaine-seeking behavior between Fisher 344 and Lewis rats. Psychopharmacol 187:22–29
Lu L, Grimm JW, Hope BT, Shaham Y (2004a) Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47(Suppl 1):214–226
Lu L, Grimm JW, Dempsey J, Shaham Y (2004b) Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology 176:101–108
Mahoney JJ, Kalechstein AD, De La Garza R, Newton TF (2007) A qualitative and quantitative review of cocaine-induced craving: the phenomenon of priming. Prog Neuropsychopharmacol Biol Psychiatry 31:593–599
Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ (2004) Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology 175:26–36
Mueller D, Stewart J (2000) Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res 115:39–47
Mueller D, Perdikaris D, Stewart J (2002) Persistence and drug-induced reinstatement of a morphine-induced conditioned place preference. Behav Brain Res 136:389–397
Ng GYK, O’Dowd BF, George SR (1994) Genotypic differences in brain dopamine receptor function in the DBA/2J and C57BL/6J inbred mouse strains. Eur J Pharmacol 269:349–64
Ng GYK, O’Dowd BF, George SR (1996) Genotypic differences in mesolimbic enkephalin gene expression in DBA/2J and C57BL/6J inbred mice. Eur J Pharmacol 311:45–52
Orsini C, Buchini F, Piazza PV, Puglisi-Allegra S, Cabib S (2004) Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology 172:264–270
Orsini C, Bonito-Oliva A, Conversi D, Cabib S (2005) Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology 181:327–336
Piazza PV, Deroche-Gamonet V, Rouge-Pont F, Le Moal M (2000) Vertical shift in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci 20:4226–4232
Puglisi-Allegra S, Cabib S (1997) Psychopharmacology of dopamine: the contribution of comparative studies in inbred strains of mice. Prog Neurobiol 51:637–661
Rescorla RA (2004) Spontaneous recovery. Learn Mem 11:501–509
Robinson TE, Kolb B (2004) Structural plasticity associated with exposure to drugs of abuse. Neuropsychopharmacology 47:33–46
Rocha BA, Odom LA, Barron BA, Ator R, Wild SA, Forster MJ (1998) Differential responsiveness to cocaine in C57BL/6J and DBA/2J mice. Psychopharmacol 138:82–88
Seale TW, Carney JM (1991) Genetic determinants of susceptibility to the rewarding and other behavioral actions of cocaine. J Addict Dis 10:141–162
Shalev U, Grimm JW, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmachol Rev 54:1–42
Sutton MA, Karanian DA, Self DW (2000) Factors that determine a propensity for cocaine-seeking behavior during abstinence in rats. Neuropsychopharmacology 22:626–41
Tran-Nguyen LTL, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL (1998) Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacol 19:48–59
van der Veen R, Piazza PV, Deroche-Gamonet V (2007) Gene-environment interactions in vulnerability to cocaine self-administration: a brief social experience affects intake in DBA/2J but not in C57BL/6J mice. Psychopharmacol 193:179–186
Ventura R, Alcaro A, Cabib S, Conversi D, Mandolesi L, Puglisi-Allegra S (2004) Dopamine in the medial prefrontal cortex controls genotype-dependent effects of amphetamine on mesoaccumbens dopamine release and locomotion. Neuropsychopharmacology 29:72–80
Acknowledgments
This research was supported by Ministero della Ricerca Scientifica e Tecnologica (COFIN: 2003053445), Ateneo 60% (2004) and Facoltà (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orsini, C., Bonito-Oliva, A., Conversi, D. et al. Genetic liability increases propensity to prime-induced reinstatement of conditioned place preference in mice exposed to low cocaine. Psychopharmacology 198, 287–296 (2008). https://doi.org/10.1007/s00213-008-1137-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1137-4