Abstract
Rationale
It has been hypothesized that sensitization of the neurochemical effects within the mesolimbic dopamine (DA) system might account for specific aspects of the addiction process. We have recently developed a self-administration procedure which produces increases in responding reinforced by cocaine on a progressive ratio (PR) schedule. This may reflect an increased motivation to self-administer cocaine, one hallmark of addiction.
Objectives
The goal of this experiment was to investigate behavioral and neurochemical changes associated with increased cocaine self-administration on a PR schedule.
Materials and methods
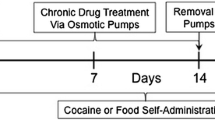
Rats self-administered cocaine over 14 days under a PR schedule. Cocaine-stimulated locomotor activity was evaluated before as well as 1 or 14 days after self-administration training. Cocaine-induced DA changes in the core and shell of the nucleus accumbens in the same animals were also examined.
Results
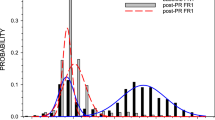
Subjects showed increased responding over time, to about 200% of baseline. Cocaine-induced locomotor activation was decreased at both withdrawal times compared to naïve animals. Microdialysis showed no differences after self-administration in the nucleus accumbens core dopamine response at either time point. There was, however, a significant decrease in the dopamine response to cocaine in the shell of the nucleus accumbens.
Conclusion
The present results demonstrate that a progressive increase in breakpoints on a PR schedule can be established in rats at a time when the ability of cocaine to increase extracellular DA levels and stimulate locomotor activity is reduced. Therefore, sensitization of the mesolimbic DA system does not account for the observed change in drug-taking behavior.




Similar content being viewed by others
References
Ahmed SH, Cador M (2006) Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology 31:563–571
Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300
Ahmed SH, Koob GF (2005) Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology 180:473–490
Ahmed SH, Lin D, Koob GF, Parsons LH (2003) Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem 86:102–113
Akimoto K, Hamamura T, Otsuki S (1989) Subchronic cocaine treatment enhances cocaine-induced dopamine efflux, studied by in vivo intracerebral dialysis. Brain Res 490:339–344
Arnold JM, Roberts DCS (1997) A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav 57:441–447
Bardo MT (1998) Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol 12:37–67
Ben Shahar O, Ahmed SH, Koob GF, Ettenberg A (2004) The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res 995:46–54
Ben Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A (2005) Prolonged daily exposure to IV cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav 82(2):411–416
Cami J, Farre M (2003) Drug addiction. N Engl J Med 349:975–986
Carlezon WA Jr, Devine DP, Wise RA (1995) Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology 122:194–197
Dackis C, O’Brien C (2005) Neurobiology of addiction: treatment and public policy ramifications. Nat Neurosci 8:1431–1436
Di Chiara G (1999) Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacol 375:13–30
Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE (2005) Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry 58:751–759
Fischman MW, Schuster CR, Javaid J, Hatano Y, Davis J (1985) Acute tolerance development to the cardiovascular and subjective effects of cocaine. J Pharm Exp Ther 235:677–682
Foltin RW, Fischman MW (1991) Smoked and intravenous cocaine in humans: acute tolerance, cardiovascular and subjective effects. J Pharmacol Exp Ther 257:247–261
Heidbreder C, Feldon J (1998) Amphetamine-induced neurochemical and locomotor responses are expressed differentially across the anteroposterior axis of the core and shell subterritories of the nucleus accumbens. Synapse 29:310–322
Hooks MS, Duffy P, Striplin C, Kalivas PW (1994) Behavioral and neurochemical sensitization following cocaine self-administration. Psychopharmacology 115:265–272
Horger BA, Shelton K, Schenk S (1990) Preexposure sensitizes rats to the rewarding effects of cocaine. Pharmacol Biochem Behav 37:707–711
Hurd YL, Weiss F, Koob GF, And NE, Ungerstedt U (1989) Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: an in vivo microdialysis study. Brain Res 498:199–203
Ikemoto S, Glazier BS, Murphy JM, McBride WJ (1997) Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci 17:8580–8587
Inada T, Polk K, Purser C, Hume A, Hoskins B, Ho IK, Rockhold RW (1992) Behavioral and neurochemical effects of continuous infusion of cocaine in rats. Neuropharmacology 31:701–708
Johanson CE (1988) Behavioral studies of the reinforcing properties of cocaine. NIDA Res Monogr 88:107–124
Kalivas PW, Duffy P (1993) Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci 13:266–275
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev 16:223–244
Kalivas PW, Churchill L, Klitenick MA (1993) Limbic motor circuits and neuropsychiatry. The circuitry mediating the translation of motivational stimuli into adaptive motor responses, pp 237–275
Koob GF, Caine B, Markou A, Pulvirenti L, Weiss F (1994) Role for the mesocortical dopamine system in the motivating effects of cocaine. NIDA Res Monogr 145:1–18
Kuczenski R, Segal DS, Todd PK (1997) Behavioral sensitization and extracellular dopamine responses to amphetamine after various treatments. Psychopharmacology 134:221–229
Lecca D, Cacciapaglia F, Valentini V, Di Chiara G (2006) Monitoring extracellular dopamine in the rat nucleus accumbens shell and core during acquisition and maintenance of intravenous WIN 55,212–2 self-administration. Psychopharmacology 188:63–74
Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G (2007) Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology 191:653–667
Liu Y, Roberts DCS, Morgan D (2005a) Effects of extended-access self-administration and deprivation on breakpoints maintained by cocaine in rats. Psychopharmacology 179:644–651
Liu Y, Roberts DCS, Morgan D (2005b) Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci 22:195–200
Lorrain DS, Arnold GM, Vezina P (2000) Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav Brain Res 107:9–19
Mateo Y, Lack CM, Morgan D, Roberts DCS, Jones SR (2005) Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology 30:1455–1463
Mendrek A, Blaha CD, Phillips AG (1998) Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology 135:416–422
Mogenson GJ, Jones DL, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14:69–97
Morgan D, Liu Y, Roberts DCS (2005) Rapid and persistent sensitization to the reinforcing effects of cocaine. Neuropsychopharmacology 31(1):121–128
Neisewander JL, O’Dell LE, Tran-Nguyen LT, Castaneda E, Fuchs RA (1996) Dopamine overflow in the nucleus accumbens during extinction and reinstatement of cocaine self-administration behavior. Neuropsychopharmacology 15:506–514
O’Brien C (2005) Drug addiction and drug abuse. In: Brunton LL, Lazo JS, Parker KL (eds) Goodman and Gilman’s the pharmacological basis of therapeutics. McGraw-Hill, New York, pp 607–627
Paterson NE, Markou A (2003) Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport 14:2229–2232
Paulson PE, Camp DM, Robinson TE (1991) Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology 103:480–492
Pettit HO, Justice JB Jr (1991) Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res 539:94–102
Pettit HO, Pan HT, Parsons LH, Justice JB Jr (1990) Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration. J Neurochem 55:798–804
Phillips AG, Di Ciano P (1996) Behavioral sensitization is induced by intravenous self-administration of cocaine by rats. Psychopharmacology 124:279–281
Pierce RC, Kalivas PW (1995) Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J Pharmacol Exp Ther 275:1019–1029
Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev 25:192–216
Post RM, Lockfeld A, Squillace KM, Contel NR (1981) Drug-environment interaction: context dependency of cocaine-induced behavioral sensitization. Life Sciences 28:755–760
Richardson NR, Roberts DCS (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11
Ridenour TA, Cottler LB, Compton WM, Spitznagel EL, Cunningham-Williams RM (2003) Is there a progression from abuse disorders to dependence disorders? Addiction 98:635–644
Roberts DCS, Corcoran ME, Fibiger HC (1977) On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav 6:615–620
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev 18:247–291
Robinson TE, Berridge KC (2000) The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 95(Suppl 2):S91–S117
Robinson TE, Berridge KC (2001) Incentive-sensitization and addiction. Addiction 96:103–114
Sellings LH, Clarke PB (2003) Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci 23:6295–6303
Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168:3–20
Sizemore GM, Cannon DG, Smith JE, Dworkin SI (2003) The effects of acutely administered cocaine on responding maintained by a progressive-ratio schedule of food presentation. Behav Pharmacol 14:33–40
Skinner BF (1938) Behavior of organisms. Appleton and Company, New York
Stewart J, Badiani A (1993) Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol 4:289–312
Swanson LW (1982) The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull 9:321–353
Thompson T (2007) Relations among functional systems in behavior analysis. J Exp Anal Behav 87:423–440
Tirelli E, Terry P (1998) Amphetamine-induced conditioned activity and sensitization: the role of habituation to the test context and the involvement of Pavlovian processes. Behav Pharmacol 9:409–419
Vezina P (2004) Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev 27:827–839
Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N (2002) Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci 22:4654–4662
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N (1997) Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386:830–833
Wagner FA, Anthony JC (2002) From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology 26:479–488
Weiner I, Gal G, Rawlins JN, Feldon J (1996) Differential involvement of the shell and core subterritories of the nucleus accumbens in latent inhibition and amphetamine-induced activity. Behav Brain Res 81:123–133
West CH, Boss-Williams KA, Weiss JM (1999) Motor activation by amphetamine infusion into nucleus accumbens core and shell subregions of rats differentially sensitive to dopaminergic drugs. Behav Brain Res 98:155–165
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psych Rev 94:469–492
Zahm DS (1999) Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci 877:113–128
Zittel-Lazarini A, Cador M, Ahmed SH (2007) A critical transition in cocaine self-administration: behavioral and neurobiological implications. Psychopharmacology 192:337–346
Acknowledgment
This study is supported by a Center Grant from the National Institute of Drug Abuse (P50-DA06634) and a National Research Service Award to CML (F30-DA06634). We declare that the experiments comply with the current laws of the USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lack, C.M., Jones, S.R. & Roberts, D.C.S. Increased breakpoints on a progressive ratio schedule reinforced by IV cocaine are associated with reduced locomotor activation and reduced dopamine efflux in nucleus accumbens shell in rats. Psychopharmacology 195, 517–525 (2008). https://doi.org/10.1007/s00213-007-0919-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0919-4