Abstract
Rationale
The rewarding effects of ethanol and other drugs of abuse are mediated by activation of the mesolimbic dopamine system. Recent neuroimaging studies in primates and humans suggest that cocaine-induced dopamine stimulation might be diminished by drugs augmenting γ-aminobutyric acid A (GABA-A) receptor function such as the GABA transaminase inhibitor vigabatrin.
Objectives
The objective of this study was to test the property of the selective GABA transporter 1 (GAT1) inhibitor tiagabine to block ethanol-induced activation of the mesolimbic reward system in an i.v. ethanol challenge.
Materials and methods
Twenty nonaddicted healthy volunteers underwent an i.v. ethanol challenge after 1 week of tiagabine (15 mg/day) administration. Neuronal activation was measured using [18F]-fluoro-deoxyglucose positron emission tomography (PET).
Results
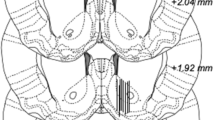
Tiagabine did not prevent ethanol-induced stimulation of the mesolimbic reward system but augmented ethanol-induced hypometabolism within areas of the visual system and the cerebellum. Tiagabine alone also decreased neuronal metabolism within parts of the right temporal cortex that are highly enriched with GABA-ergic neurons.
Conclusions
Our ethanol challenge imaging study does not provide supporting evidence that the GAT1 inhibitor tiagabine diminishes the rewarding effects of ethanol. Further PET imaging studies using established anticraving compounds, such as the μ-opioid receptor antagonist naltrexone and antiepileptic drugs affecting the GABA-ergic system more broadly, will provide additional important insights on the interaction between the GABA-ergic and the brain reward system in vivo and the suitability of GABA-ergic drugs as anticraving compounds.

Similar content being viewed by others
References
Anand A, Shekhar A (2003) Brain imaging studies in mood and anxiety disorders: special emphasis on the amygdala. Ann N Y Acad Sci 985:370–388
Bartlett EJ, Brodie JD, Wolf AP, Christman DR, Laska E, Meissner M (1988) Reproducibility of cerebral glucose metabolic measurements in resting human subjects. J Cereb Blood Flow Metab 8:502–512
Berke JD, Hyman SE (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25:515–532
Boehm SL 2nd, Piercy MM, Bergstrom HC, Phillips TJ (2002) Ventral tegmental area region governs GABA(B) receptor modulation of ethanol-stimulated activity in mice. Neuroscience 115:185–200
Brodie JD, Figueroa E, Dewey SL (2003) Treating cocaine addiction: from preclinical to clinical trial experience with gamma-vinyl GABA. Synapse 50:261–265
Connelly JF (1993) Vigabatrin. Ann Pharmacother 27:197–204
Dalby NO (2000) GABA-level increasing and anticonvulsant effects of three different GABA uptake inhibitors. Neuropharmacology 39:2399–2407
Davidson RJ (2002) Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 51:68–80
Dewey SL, Morgan AE, Ashby CR Jr, Horan B, Kushner SA, Logan J, Volkow ND, Fowler JS, Gardner EL, Brodie JD (1998) A novel strategy for the treatment of cocaine addiction. Synapse 30:119–129
Dewey SL, Brodie JD, Gerasimov M, Horan B, Gardner EL, Ashby CR Jr (1999) A pharmacologic strategy for the treatment of nicotine addiction. Synapse 31:76–86
Eriksson IS, Allard P, Marcusson J (1999) [3H]tiagabine binding to GABA uptake sites in human brain. Brain Res 851:183–188
Friston KJ, Frith CD, Liddle PF, Dolan RJ, Lammertsma AA, Frackowiak RS (1990) The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab 10:458–466
Friston KJ, Frith CD, Liddle PF, Frackowiak RS (1991) Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 11:690–699
Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ (1995a) Spatial registration and normalization of images. Hum Brain Mapp 3:165–189
Friston KJ, Holmes AP, Worsley K, Poline JB, Frith CD, Frankowiak RSJ (1995b) Statistical parametric maps in functional brain imaging: a general linear approach. Hum Brain Mapp 2:189–210
Gentry JR, Hill C, Malcolm R (2002) New anticonvulsants: a review of applications for the management of substance abuse disorders. Ann Clin Psychiatry 14:233–245
Gerasimov MR, Ashby CR Jr, Gardner EL, Mills MJ, Brodie JD, Dewey SL (1999) Gamma-vinyl GABA inhibits methamphetamine, heroin, or ethanol-induced increases in nucleus accumbens dopamine. Synapse 34:11–19
Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652
Gonzales RA, Weiss F (1998) Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 18:10663–10671
Gonzalez G, Sevarino K, Sofuoglu M, Poling J, Oliveto A, Gonsai K, George TP, Kosten TR (2003) Tiagabine increases cocaine-free urines in cocaine-dependent methadone-treated patients: results of a randomized pilot study. Addiction 98:1625–1632
Hachiya Y, Takashima S (2001) Development of GABAergic neurons and their transporter in human temporal cortex. Pediatr Neurol 25:390–396
Harris RA (1999) Ethanol actions on multiple ion channels: which are important? Alcohol Clin Exp Res 23:1563–1570
Johannessen SI, Battino D, Berry DJ, Bialer M, Kramer G, Tomson T, Patsalos PN (2003) Therapeutic drug monitoring of the newer antiepileptic drugs. Ther Drug Monit 25:347–363
Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413
Kalivas PW, Duffy P, Eberhardt H (1990) Modulation of A10 dopamine neurons by gamma-aminobutyric acid agonists. J Pharmacol Exp Ther 253:858–866
Kastberg H, Jansen JA, Cole G, Wesnes K (1998) Tiagabine: absence of kinetic or dynamic interactions with ethanol. Drug Metab Drug Interac 14:259–273
Koob GF, Le Moal M (2005) Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 8:1442–1444
Kumar S, Fleming RL, Morrow AL (2004) Ethanol regulation of gamma-aminobutyric acid A receptors: genomic and nongenomic mechanisms. Pharmacol Ther 101:211–226
LaRoche SM, Helmers SL (2004) The new antiepileptic drugs: clinical applications. JAMA 291:615–620
Lile JA, Stoops WW, Glaser PE, Hays LR, Rush CR (2004) Acute administration of the GABA reuptake inhibitor tiagabine does not alter the effects of oral cocaine in humans. Drug Alcohol Depend 76:81–91
Lumer ED, Friston KJ, Rees G (1998) Neural correlates of perceptual rivalry in the human brain. Science 280:1930–1934
Malcolm RJ (2003) GABA systems, benzodiazepines, and substance dependence. J Clin Psychiatry 64(Suppl 3):36–40
Martin DL, Rimvall K (1993) Regulation of gamma-aminobutyric acid synthesis in the brain. J Neurochem 60:395–407
Mavridis M, Besson MJ (1999) Dopamine–opiate interaction in the regulation of neostriatal and pallidal neuronal activity as assessed by opioid precursor peptides and glutamate decarboxylase messenger RNA expression. Neuroscience 92:945–966
Meldrum BS, Chapman AG (1999) Basic mechanisms of gabitril (tiagabine) and future potential developments. Epilepsia 40(Suppl 9):S2–S6
Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL (1997) Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389:385–389
Nguyen SA, Deleon CP, Malcolm RJ, Middaugh LD (2005) Tiagabine reduces ethanol reward in C57BL/6 mice under acute and chronic administration regimens. Synapse 56:135–146
Rosenthal M (2003) Tiagabine for the treatment of generalized anxiety disorder: a randomized, open-label, clinical trial with paroxetine as a positive control. J Clin Psychiatry 64:1245–1249
Rustum AM, Estrada V (1998) Separation and quantitation of the S-(+)-enantiomer in the bulk drug tiagabine × HCl by chiral high-performance-liquid chromatography using a Chiralcel-OD column. J Chromatogr B Biomed Sci Appl 705:111–117
Sarter M, Givens B, Bruno JP (2001) The cognitive neuroscience of sustained attention: where top–down meets bottom–up. Brain Res Brain Res Rev 35:146–160
Sarter M, Gehring WJ, Kozak R (2006) More attention must be paid: the neurobiology of attentional effort. Brain Res Brain Res Rev 51:145–160
Schiffer WK, Gerasimov MR, Bermel RA, Brodie JD, Dewey SL (2000) Stereoselective inhibition of dopaminergic activity by gamma vinyl-GABA following a nicotine or cocaine challenge: a PET/microdialysis study. Life Sci 66:PL169–PL173
Schiffer WK, Marsteller D, Dewey SL (2003) Sub-chronic low dose gamma-vinyl GABA (vigabatrin) inhibits cocaine-induced increases in nucleus accumbens dopamine. Psychopharmacology (Berl) 168:339–343
Schmitt U, Luddens H, Hiemke C (2002) Anxiolytic-like effects of acute and chronic GABA transporter inhibition in rats. J Neural Transm 109:871–880
Schreckenberger M, Amberg R, Scheurich A, Lochmann M, Tichy W, Klega A, Siessmeier T, Gründer G, Buchholz HG, Landvogt C, Stauss J, Mann K, Bartenstein P, Urban R (2004a) Acute alcohol effects on neuronal and attentional processing: striatal reward system and inhibitory sensory interactions under acute ethanol challenge. Neuropsychopharmacology 29:1527–1537
Schreckenberger M, Lange-Asschenfeld C, Lochmann M, Mann K, Siessmeier T, Buchholz HG, Bartenstein P, Gründer G (2004b) The thalamus as the generator and modulator of EEG alpha rhythm: a combined PET/EEG study with lorazepam challenge in humans. Neuroimage 22:637–644
Stanford IM, Cooper AJ (1999) Presynaptic mu and delta opioid receptor modulation of GABAA IPSCs in the rat globus pallidus in vitro. J Neurosci 19:4796–4803
Stief F, Piechotta A, Gabriel S, Schmitz D, Draguhn A (2005) Functional GABA uptake at inhibitory synapses in CA1 of chronically epileptic rats. Epilepsy Res 66:199–202
Swan M, Najlerahim A, Watson RE, Bennett JP (1994) Distribution of mRNA for the GABA transporter GAT-1 in the rat brain: evidence that GABA uptake is not limited to presynaptic neurons. J Anat 185(Pt 2):315–323
Takayama C, Inoue Y (2005) Developmental expression of GABA transporter-1 and 3 during formation of the GABAergic synapses in the mouse cerebellar cortex. Brain Res Dev Brain Res 158:41–49
Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Montgomery MA, Goldsmith RJ, Coleman FS, Bloch DA, Leiderman DB, Singal BM, Berger P, Elkashef A (2005) A placebo-controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction 100(Suppl 1):68–77
Woodward JJ, Ron D, Winder D, Roberto M (2006) From blue states to up states: a regional view of NMDA–ethanol interactions. Alcohol Clin Exp Res 30:359–367
Worsley KJ, Cao J, Paus T, Petrides M, Evans AC (1998) Applications of random field theory to functional connectivity. Hum Brain Mapp 6:364–367
Acknowledgment
This study was supported by the MAIFOR program of the University of Mainz. Some of the results of this article form a part of the physician’s thesis of the coauthor Natalie Chechko at the Medical Faculty of the Johannes Gutenberg-University Mainz, Germany. Furthermore, the authors thank Mrs. Uta Juergens, Epilepsy Research Center, Kehl-Kork, Germany, for measuring the tiagabine plasma levels.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fehr, C., Hohmann, N., Gründer, G. et al. Tiagabine does not attenuate alcohol-induced activation of the human reward system. Psychopharmacology 191, 975–983 (2007). https://doi.org/10.1007/s00213-006-0696-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0696-5