Abstract
Rationale
Functional magnetic resonance imaging (fMRI) in rats can non-invasively identify brain regions activated by physiological stimuli and the effects of pharmacological intervention on these responses.
Objectives
This study was conducted to investigate the effects of systemic administration of the μ-opioid receptor agonist morphine on whole brain functional signal intensity in anaesthetised rats; to investigate whether pre-treatment with the opioid receptor antagonist naloxone blocks the effects of morphine; to determine whether pre-treatment with morphine attenuates noxious-evoked changes in whole brain functional signal intensity.
Methods
Continuous whole brain fMRI scanning was used to study brain signal intensity prior to, and following, systemic administration of morphine (5 mg/kg, n=7), systemic administration of naloxone (1 mg/kg) and morphine (n=8). Effects of pre-treatment with saline (n=5) or morphine (5 mg/kg, n=5) on formalin (5%, intraplantar)-evoked changes in signal intensity were determined. Data were processed using SMP99 with fixed-effects analysis (p<0.05).
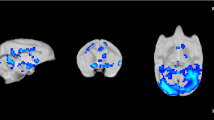
Results
Morphine produced significant positive bilateral increases in signal intensity in the cingulate cortex, amygdala, thalamus, hypothalamus and PAG (p<0.05), and these effects were blocked by naloxone. Intraplantar injection of formalin produced a significant positive increase in signal intensity in the cingulate cortex, somatosensory cortex, amygdala, thalamus, hypothalamus and PAG (p<0.05). Morphine attenuated formalin-evoked increases in signal intensity in the PAG, amygdala, hypothalamus and cingulate cortex.
Conclusion
Our data demonstrate that morphine modulates noxious-evoked changes in signal intensity in discrete brain regions. fMRI studies in rats are able to identify specific brain regions involved in the pharmacological modification of physiologically evoked changes in regional brain activation.







Similar content being viewed by others
Abbreviations
- BOLD:
-
Blood oxygenation level dependent
- Cingulate CTX:
-
Cingulate cortex
- DlPAG:
-
Dorsal lateral periaqueductal grey
- DmCPU:
-
Dorsal medial caudate putamen
- fMRI:
-
Functional magnetic resonance imaging
- HindSSCTX:
-
Hindlimb area of somatosensory cortex
- Lhypo:
-
Lateral hypothalamus
- MR:
-
Magnetic resonance
- MAP:
-
Mean arterial blood pressure
- RARE:
-
Rapid acquisition relaxation enhanced
- rCBF:
-
Regional cerebral bloodflow
- RF:
-
Radiofrequency
- SC:
-
Subcutaneous
- SST:
-
Spinal thalamic tract
- VTA:
-
Ventral tegmental area
References
Aloisi AM, Porro CA, Cavazzuti M, Baraldi P, Carli G (1993) Mirror pain in the formalin test: behavioural and 2-deoxyglucose studies. Pain 55:267–273
Baulmann J, Spitznagel H, Herdegen T, Unger T, Culman J (2000) Tachykinin receptor inhibition and c-Fos expression in the rat brain following formalin-induced pain. Neuroscience 95:813–820
Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D (1999) Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Reson Med 41:1044–1057
Bernard JF, Bester H, Besson JM (1995) The spino-parabrachio-amygdaloid and hypothalamic nociceptive pathways. In: Besson JM, Guilbaud G, Ollat H (eds) Forebrain areas involved in pain processing. John Libbey Eurotext, France, pp 27–49
Borras MC, Becerra L, Ploghaus A, Gostic JM, Dasilva A, Gonzalez RG, Borsook D (2004) FMRI measurement of CNS responses to naloxone infusion and subsequent mild noxious thermal stimuli in healthy volunteers. J Neurophysiol 91:2723–2733
Bourgeais L, Monconduit L, Villanueva L, Barnard J-F (2001a) Parabrachial internal lateral neurons convey nociceptive messages from the deep laminas of the dorsal horn to the intralaminar thalamus. J Neurosci 21:2159–2165
Bourgeais L, Gauriau C, Barnard JF (2001b) Projections from the noccieptive area of the central nucleus of the amygdala to the forebrain: a PHA-L study in the rat. Eur J Neurosci 14:229–255
Casey KL (1999) Forebrain mechanisms of nociception and pain: analysis through imaging. Proc Natl Acad Sci U S A 96:7668–7674
Casey KL (2000) Concepts of pain mechanisms: the contribution of functional imaging of the human brain. Prog Brain Res 129:277–287
Chang C, Shyu B-C (2001) A fMRI study of brain activations during non-noxious and noxious electrical stimulation of the sciatic nerve of rats. Brain Res 897:71–81
Cohen SR, Melzack R (1985) Morphine injected into the habenula and dorsal posteromedial thalamus produces analgesia in the formalin test. Brain Res 359:131–139
Cohen SR, Kimes AS, London ED (1991) Morphine decrease cerebral glucose utilization in limbic and forebrain regions while pain has no effect. Neuropharmacology 30:125–134
Dafny N, Marchand J, McClung R, Salamy J, Sands S, Wachtendorf H, Burks TF (1980) Effects of morphine on sensory-evoked responses recorded from central gray, reticular formation, thalamus, hypothalamus, limbic system, basal ganglia, dorsal raphe, locus ceruleus, pineal body. J Neurosci Res 5:399–412
Dubuisson D, Dennis SG (1977) The formalin test: a quantitative study of the analgesic effects of morphine, meperdine, and brain stem stimulation in rats and cats. Pain 4:161–174
Fields H (2000) Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res 122:245–253
Friston KJ, Ashburner J, Poline JB, Frith CD, Heather JD, Frackowiak RSJ (1995a) Spatial registration and normalization of images. Hum Brain Mapp 2:165–189
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ (1995b) Human Brain Mapping 1:214–220 [SPM_3] Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2:189–210
Gauriau C, Bernard JF (2004) Pain pathways and parabrachial circuits in the rat. Exp Physiol 87:251–258
Gebhart GF (2004) Descending modulation of pain. Neurosci Biobehav Rev 27:729–737
Guilbaud G, Peschanski M, Gautron M, Binder D (1980) Neurones responding to noxious stimulation in VB complex and caudal adjacent regions in the thalamus of the rat. Pain 8:303–318
Haley JE, Sullivan AF, Dickenson AH (1990) Evidence for the N-methyl-d-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res 518:218–226
Hennig J, Nauerth A, Friedburg H (1986) RARE imaging: a fast imaging method for clinical MR. Magn Reson Med 3:823–833
Hill RG, Salt TE, Pepper CM (1982) A comparison of the effectiveness of intravenous morphine at attenuating the nociceptive responses of medullary dorsal horn and thalamic neurones. Life Sci 31:2331–2334
Johnson SW, North RA (1992) Opioids excite dopamine neurones by hyperpolarisation of local interneurones. J Neurosci 12:483–488
Jones AK, Luthra SK, Maziere B, Pike VW, Loc’h C, Crouzel C, Syrota A, Jones T (1988) Regional cerebral opioid receptor studies with [11C]diprenorphine in normal volunteers. J Neurosci Methods 23:121–129
Jones AK, Cunningham VJ, Ha-Kawa S, Fujowara T, Luthra SK, Silva S, Derbyshire S, Jones T (1994) Changes in central opioid receptor binding in relation to inflammation and pain in patients with rheumatoid arthritis. Br J Rheumatol 33:909–916
Keilholz SD, Silva AC, Raman M, Merkle H, Koretsky AP (2004) Functional MRI of the rodent somatosensory pathway using multislice echo planar imaging. Magn Reson Med 52:89–99
LeBrun P, Manil J, Colin F (2000) Formalin-induced central sensitization in the rat: somatosensory evoked potential data. Neurosci Lett 283:113–116
Leslie RA, James MF (2000) Pharmacological magnetic resonance imaging: a new application for functional MRI. TIPS 21:314–318
Lewis VA, Gebhart GF (1977) Evaluation of the periaqueductal central gray (PAG) as a morphine-specific locus of action and examination of morphine-induced and stimulation-produced analgesia at coincident PAG loci. Brain Res 124:283–303
Luo F, Wu G, Li Z, Li S-J (2003) Characterization of effects of mean arterial blood pressure induced by cocaine and cocaine methiodide on BOLD signals in rat brain. Magn Reson Med 49:264–270
Malisza K, Docherty JC (2001) Capsaicin as a source of painful stimulation in functional MRI. J Magn Reson Imaging 14:341–347
Malisza K, Frankenstein U, Stroman P, Docherty J (2001) Negative functional MRI changes in capsaicin-induced painful stimulation in rats. Proc Intl Soc Mag Reson Med 9:657
Manning BH, Franklin KB (1998) Morphine analgesia in the formalin test: reversal by microinjection of quaternary naloxone into the posterior hypothalamic area or periaqueductal gray. Behav Brain Res 92:97–102
Manning BH, Mayer DJ (1995) The central nucleus of the amygdala contributes to the production of morphine antinociception in the formalin test. Pain 63:141–152
Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ (1987) Autoradiographic differentiation of mu, delta, kappa and opioid receptors in the rat forebrain and midbrain. J Neurosci 7:2445–2464
Matos FF, Rollema H, Taiwo YO, Levine JD, Basbaum AI (1995) Relationship between analgesia and extracellular morphine in brain and spinal cord in awake rats. Brain Res 693:187–195
Morrow TJ, Paulson PE, Danneman PJ, Casey KL (1998) Regional changes in forebrain activation during the early and late phase of formalin nociception: analysis using cerebral blood flow in the rat. Pain 75:355–365
Petrovic P, Kalso E, Petersson KM, Ingvar M (2002) Placebo and opioid analgesia—imaging a shared neuronal network. Science 295:1737–1740
Porro CA, Cavazzuti M, Galetti A, Sassatelli L (1991) Functional activity mapping of the rat brainstem during formalin-induced noxious stimulation. Neuroscience 41:667–680
Porro CA, Cavazzuti M, Baraldi P, Giuliani D, Panerai AE, Corazza R (1999) CNS pattern of metabolic activity during tonic pain: evidence for modulation by β-endorphin. Eur J Neurosci 11:874–888
Presley RW, Menetrey D, Levine JD, Basbaum (1990) Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J Neurosci 10:323–335
Puig S, Sorkin LS (1996) Formalin-evoked activity in identified primary afferent fibre systemic lidocaine suppresses phase 2 activity. Pain 64:345–355
Schlaepfer TE, Strain EC, Greenberg BD, Preston KL, Lancasyer E, Bigelow GE, Barta PE, Pearlson GD (1998) Site of opioid action in the human brain: mu and kappa agonits’ subjective and cerebral blood flow effects. Am J Psychiatry 155:470–473
Shah YB, Prior MJW, Dixon AL, Morris PG, Marsden CA (2004) Detection of cannabinoid agonist evoked increase in BOLD contrast in rats using functional magnetic resonance imaging. Neuropharmacology 46:379–387
Tracey I, Becerra L, Chang I, Breiter H, Jenkins L, Borsook D, Gonzalez RG (2000) Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci Lett 288:159–162
Tuor UI, Malisza K, Foniok T, Papadimiitropoulos R, Jarmasz M, Somorjai R, Kozlowski P (2000) Functional magnetic resonance imaging in rats subjected to intense electrical and noxious chemical stimulation of the forepaw. Pain 87:315–324
Tuor UI, McKenzie E, Tomanek B (2002) Functional magnetic resonance imaging of tonic pain and vasopressor effects in rats. Magn Reson Imaging 20:707–712
Willis WD (1989) The origin and destination of pathways involved in pain transmission. In: Wall P, Melzack R (eds) The textbook of pain. Churchill Livingstone, London, pp 112–128
Wise RG, Rogers R, Painter D, Bantick S, Ploghaus A, Williams P, Rapeport G, Tracey I (2002) Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. NeuroImage 16:999–1014
Wise RG, Willimas P, Tracey I (2004) Using fMRI to quantify the time dependence of remifentanil analgesia in the human brain. Neurophsychopharmacology 29:626–635
Yaksh TL, Rudy TA (1977) Studies on the direct spinal action of narcotics in the production of analgesia in the rat. J Pharmacol Exp Ther 202:411–428
Yang ZJ, Tang JS, Jia H (2002) Morphine microinjections into the rat nucleus submedius depress nociceptive behaviour in the formalin test. Neurosci Lett 328:141–144
Yeung JC, Yaksh TL, Rudy TA (1977) Concurrent mapping of brain sites for sensitivity to the direct application of morphine and focal electrical stimulation in the production of antinociception in the rat. Pain 4:23–40
Zubieta J-K, Smith Y, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS (2001) Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 293:311–315
Acknowledgements
The authors thank Dr. M Randall for his help in performing measurements of blood pressure. This project was supported by Merck and benefited from an MRC JREI grant in conjunction with AstraZeneca
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shah, Y.B., Haynes, L., Prior, M.J.W. et al. Functional magnetic resonance imaging studies of opioid receptor-mediated modulation of noxious-evoked BOLD contrast in rats. Psychopharmacology 180, 761–773 (2005). https://doi.org/10.1007/s00213-005-2214-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-2214-6