Abstract
Rationale
Ultra-low-dose opioid antagonists enhance opiate analgesia and attenuate tolerance and withdrawal.
Objectives
To determine whether ultra-low-dose naltrexone (NTX) coadministration alters the rewarding effects of opiates or the aversive effects of opiate withdrawal.
Methods
We used the conditioned place preference (CPP) and conditioned place aversion (CPA) paradigms to assess whether ultra-low-dose NTX alters the acute rewarding effects of oxycodone or morphine, or the aversive aspect of withdrawal from either drug. To assess the dose response for ultra-low-dose NTX, a range of NTX doses (0.03–30 ng/kg) was tested in the oxycodone CPP experiment. In order to avoid tolerance or sensitization effects, we used single conditioning sessions and female rats, as females are more sensitive to the conditioning effects of these drugs.
Results
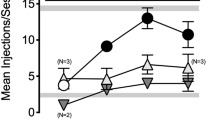
Ultra-low-dose NTX (5 ng/kg) blocked the CPP to morphine (5 mg/kg) and the CPA to withdrawal from chronic morphine (5 mg/kg, for 7 days). Coadministration of ultra-low-dose NTX (30 pg/kg) also blocked the CPA to withdrawal from chronic oxycodone administration (3 mg/kg, for 7 days). The effects of NTX on the CPP to oxycodone (3 mg/kg) revealed a biphasic dose response. The two lowest doses (0.03 and 0.3 ng/kg) blocked the CPP, the middle dose (3 ng/kg) was ineffective, and oxycodone combined with the highest dose (30 ng/kg) produced a trend toward a CPP.
Conclusions
Ultra-low-dose NTX coadministration blocks the acute rewarding effects of analgesic doses of oxycodone or morphine as well as the anhedonia of withdrawal from chronic administration.




Similar content being viewed by others
References
Azar MR, Jones BC, Shulteis G (2003) Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology 170:42–50
Bechara A, Nader K, van der Kooy D (1995) Neurobiology of withdrawal motivation: evidence for two separate aversive effects produced in morphine-naive versus morphine-dependent rats by both naloxone and spontaneous withdrawal. Behav Neurosci 109:91–105
Bowman BP, Vaughn S, Walker Q, Davis S, Little PSN, Thomas B, Kuhn CM (1999) Effect of gender and gonadectomy on cocaine metabolism in rat. J Pharmacol Exp Ther 290:1316–1323
Chen H, Yang Y, Yeh T, Cherng C, Hsu H, Hsiao S, Yu L (2003) Methamphetamine-induced conditioned place preference is facilitated by estradiol pretreatment in female mice. Chin J Physiol 46:169–174
Chindalore VL, Butera PG, Yu KP, Burns LH, Friedmann N (2005) Adding ultra-low-dose naltrexone to oxycodone enhances and prolongs analgesia. J Pain 6:392–399
Crain SM, Shen K-F (1995) Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its antinociceptive potency and attenuating tolerance/dependence during chronic cotreatment. Proc Natl Acad Sci U S A 92:10540–10544
Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V (2004) Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 46:672–687
Franklin KB (1998) Analgesia and abuse potential: an accidental association or a common substrate? Pharmacol Biochem Behav 59:993–1002
Kest B, Sarton E, Dahan A (2000) Gender differences in opioid-mediated analgesia. Anesthesiology 93:539–547
Koob GF, Stinus L, Le Moal M, Bloom FE (1989) Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev 13:135–140
Leri F, Burns LH (2005) Ultra-low-dose naltrexone decreases rewarding potency and the vulnerability to relapse in the reinstatement model of drug relapse. Pharmacol Biochem Behav (in press)
Mansour A, Fox CA, Akil H, Watson SJ (1995) Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci 18:22–29
Nazarian A, Russo S, Festa E, Kraish M, Quinones-Jenab V (2004) The role of D(1) and D(2) receptors in the cocaine conditioned place preference of male and female rats. Brain Res Bull 63:295–299
Powell KJ, Abul-Husn NS, Jhamandas A, Olmstead MC, Beninger RJ, Jhamandas K (2002) Paradoxical effects of the opioid antagonist naltrexone on morphine analgesia, tolerance, and reward in rats. J Pharmacol Exp Ther 300:588–596
Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T (1994) Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol 45:330–334
Shen KF, Crain SM (1994) Antagonists at excitatory opioid receptors on sensory neurons in culture increase potency and specificity of opiate analgesics and attenuate development of tolerance/dependence. Brain Res 636:286–297
Shen K-F, Crain SM, Moate P, Boston R, de Kater AW, Schoenhard GL (2002a) PTI-555, reverses and prevents morphine-induced tolerance and naloxone-precipitated withdrawal in mice chronically treated with morphine. Pain 3(suppl 1):50
Shen K-F, Crain SM, Moate P, Boston R, de Kater AW, Schoenhard GL (2002b) PTI-801, a novel formulation of oxycodone, shows absence of tolerance, physical dependence and naloxone-precipitated withdrawal effects in mice. Pain 3(suppl 1):49
Wang H-Y, Friedman E, Olmstead MC, Burns LH (2005) Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in Mu opioid receptor-G protein coupling and Gβγ signaling. Neuroscience (in press)
Acknowledgements
The technical assistance of Jay Paquette and Stephanie Hancock is gratefully acknowledged. This work was funded by Pain Therapeutics, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olmstead, M.C., Burns, L.H. Ultra-low-dose naltrexone suppresses rewarding effects of opiates and aversive effects of opiate withdrawal in rats. Psychopharmacology 181, 576–581 (2005). https://doi.org/10.1007/s00213-005-0022-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0022-7