Abstract
Rationale
Previous work has shown that a single exposure of rats to a severe stressor (immobilization, IMO) results, days to weeks later, in a reduced response (desensitization) of the hypothalamic–pituitary–adrenal (HPA) axis to a second exposure to the same stressor.
Objectives
In the present work, we studied the influence of both length of exposure to IMO and circulating levels of corticosterone on the first day on the degree of desensitization of two sets of physiological variables: HPA hormones and food intake.
Methods
Rats were given SC saline or ACTH administration and then exposed to IMO for 0, 1 or 20 min. Seven days later, all rats were exposed to 20 min IMO. HPA response was followed on both experimental days by repeated blood sampling and food intake was measured on a 24-h basis.
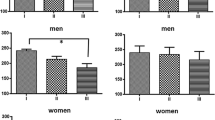
Results
Both ACTH administration and IMO activates the HPA axis and IMO reduced food intake for several days. A single previous experience with IMO enhanced the post-IMO return of HPA hormones to basal levels on day 8 and reduced the degree of anorexia. The protective effect of previous IMO on food intake was independent of, whereas that on HPA activation was positively related to, the length of exposure on day 1. Concomitant ACTH administration on day 1 did not modify the observed effects.
Conclusions
Long-term protective effects of a single exposure to IMO are observed even with a brief exposure, but they are not potentiated by increasing corticosterone levels during the first exposure.




Similar content being viewed by others
References
Adamec R, Shallow T (1993) Lasting effects on rodent anxiety of a single exposure to a cat. Physiol Behav 54:101–109
Antelman SM, Knopf S, Kocan D, Edwards DJ, Ritchie JC, Nemeroff CB (1988) One stressful event blocks multiple actions of diazepam for up to at least a month. Brain Res 445:380–385
Caggiula AR, Antelman SM, Aul E, Knopf S, Edwards DJ (1989) Prior stress attenuates the analgesic response but sensitizes the corticosterone and cortical dopamine responses to stress 10 days later. Psychopharmacology 99:233–237
Carobrez SG, Gasparotto OC, Buwalda B, Bohus B (2002) Long-term consequences of social stress on corticosterone and IL-1β levels in endotoxin-challenged rats. Physiol Behav 76:99–105
Cordero MI, Sandi C (1998) A role for brain glucocorticoid receptors in contextual fear conditioning: dependence upon training intensity. Brain Res 786:11–17
Dal-Zotto S, Martí O, Armario A (2002) Is repeated exposure to immobilization needed to induce adaptation of the hypothalamic-pituitary-adrenal axis? Influence of adrenal factors. Behav Brain Res 129:187–195
Dal-Zotto S, Martí O, Armario A (2003) Glucocorticoids are involved in the long-term effects of a single immobilzation stress on the hypothalamic–pituitary–adrenal axis. Psychoneuroendocrinology 28:992–1009
García A, Martí O, Dal-Zotto S, Vallès A, Armario A (2000) Recovery of the hypothalamic–pituitary–adrenal response to stress: effect of stress intensity, stress duration and previous stress exposure. Neuroendocrinology 72:114–125
Groves PM, Thompson RF (1970) Habituation: a dual-process theory. Psychol Rev 77:419–450
Hayley S, Brebner K, Lacosta S, Merali Z, Anisman H (1999) Sensitization to the effects of tumor necrosis factor-α: neuroendocrine, central monoamine, and behavioral variations. J Neurosci 19:5654–5665
Hayley S, Lacosta S, Merali Z, van Rooijen N, Anisman H (2001) Central monoamine and plasma corticosterone changes induced by a bacterial endotoxin: sensitization and cross-sensitization effects. Eur J Neurosci 13:1155–1165
Hayley S, Wall P, Anisman H (2002) Sensitization to the neuroendocrine, central monoamine and behavioural effects of murine tumor necrosis factor-α: peripheral and central mechanisms. Eur J Neurosci 15:1061–1076
Herman JP, Cullinan WE (1997) Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurol Sci 20:78–84
Johnson JD, O’Connor KA, Deak T, Spencer RL, Watkins LR, Maier SF (2002) Prior stressor exposure primes the HPA axis. Psychoneuroendocrinology 27:353–365
Koolhaas JM, Hermann PM, Kemperman C, Bohus B, van den Hoofdakker RH, Beersma DGM (1990) Single social defeat in male rats induces a gradual but long lasting behavioural change: a model of depression? Neurosci Res Commun 7:35–41
Kovacs KJ (1998) c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int 33:287–297
Lahmame A, Gómez F, Armario A (1996) Fawn-hooded rats show enhanced active behaviour in the forced swimming test, with no evidence for pituitary–adrenal hyperactivity. Psychopharmacology 125:74–78
Liberzon I, Krstov M, Young EA (1997) Stress–restress: effects on ACTH and fast feedback. Psychoneuroendocrinology 22:443–453
Lupien SJ, McEwen BS (1997) The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Rev 24:1–27
Maier SF (1993) Learned helplessness: relationships with fear and anxiety. In: Stanford SC, Salmon P (eds) Stress. From synapse to syndrome. Academic, London, pp 207–243
Márquez C, Belda X, Armario A (2002) Post-stress recovery of pituitary-adrenal hormones and glucose, but not the response during exposure to the stressor, is a marker of stress intensity in highly stressful situations. Brain Res 926:181–185
Martí O, Martí J, Armario A (1994) Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav 55:747–753
Martí O, García A, Vallès A, Harbuz MS, Armario A (2001) Evidence that a single exposure to aversive stimuli triggers long-lasting effects in the hypothalamus–pituitary–adrenal axis that consolidate with time. Eur J Neurosci 13:1–10
McGaugh JL (2000) Memory—a century of consolidation. Science 287:248–251
Nader K, Schafe GE, Le Doux JE (2000) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406:722–726
O’Connor KA, Johnson JD, Hammack SE, Brooks LM, Spencer RL, Watkins LR, Maier SF (2003) Inescapable shock induces resistance to the effects of dexamethasone. Psychoneuroendocrinology 28:481–500
Pacak K, Palkovits M (2001) Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 22:502–548
Roozendaal B (2000) Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology 25:213–238
Schmidt ED, Janszen AWJW, Wouterlood FG, Tilders FJH (1995) Interleukin-1-induced long-lasting changes in hypothalamic corticotropin-releasing hormone (CRH)-neurons and hyperresponsiveness of the hypothalamus–pituitary–adrenal axis. J Neurosci 15:7417–7426
Schmidt ED, Janszen AWJW, Binnekade R, Tilders FJH (1997) Transient suppression of resting corticosterone levels induces sustained increase of AVP stores in hypothalamic CRH-neurons of rats. J Neuroendocrinol 9:69–77
Schmidt ED, Tilders FJH, Binnekade R, Schoffelmeer NM, De Vries TJ (1999) Stressor- or drug-induced sensitization of the corticosterone response is not critically involved in the long-term expression of behavioural sensitization to amphetamine. Neuroscience 92:343–352
Schmidt ED, Schoffelmeer NM, De Vries TJ, Wardeh G, Dogterom G, Bol JGJM, Binnekade R Tilders FJH (2001) A single administration of interleukin-1 or amphetamine induces long-lasting increases in evoked noradrenaline release in the hypothalamus and sensitization of ACTH and corticosterone responses in rats. Eur J Neurosci 13:1923–1930
Vallès A, Martí O, García A, Armario A (2000) Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am J Physiol 279:R1138–R1144
Vallès A, Martí O, Armario A (2003) Long-term effects of a single exposure to immobilization stress on the hypothalamic–pituitary–adrenal axis: trancriptional evidence for a progressive desensitization process. Eur J Neurosci 18:1353–1361
Vanderschuren LJMJ, Schmidt ED, De Vries TJ, Van Moorsel CAP, Tilders FJH, Schofelmeer ANM (1999) A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neurosci 19:9579–9586
Van Dijken HH, de Goeij DCE, Sutanto W, Mos J, de Kloet ER, Tilders FJH (1993) Short inescapable stress produces long-lasting changes in the brain–pituitary–adrenal axis of adult male rats. Neuroendocrinology 58:57–64
Acknowledgements
This work was supported by grants from DGICYT (PM98-175 and SAF2002-623) and CUR (1999SGR-330). Silvina Dal-Zotto was a fellowship of the Agencia Española de Cooperación Internacional. We thank Dr. W.C. Engeland (Department of Surgery, University of Minnesota, Minn., USA) for his gift of the ACTH antiserum.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dal-Zotto, S., Martí, O., Delgado, R. et al. Potentiation of glucocorticoid release does not modify the long-term effects of a single exposure to immobilization stress. Psychopharmacology 177, 230–237 (2004). https://doi.org/10.1007/s00213-004-1939-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-004-1939-y