Abstract
Rationale
Sensorimotor gating disruption is one of many neurocognitive deficits seen in schizophrenia. Disorganized thought is one of the cardinal symptoms associated with sensorimotor gating. In an attempt to model sensorimotor gating deficits in rats relevant to the neurodevelopmental hypothesis for schizophrenia, we have used prenatal injections of the antimitotic drug, cytosine arabinoside (Ara-C) to subtly perturb the development of the rat CNS and disrupt sensorimotor gating.
Objective
To produce rats with either basal sensorimotor gating deficits or increased vulnerability to the disruption of sensorimotor function by apomorphine or phencyclidine (PCP). Prepulse inhibition (PPI) of the acoustic startle response was used to assess sensorimotor gating.
Methods
Three different cohorts of pregnant Sprague Dawley female rats were injected with Ara-C (30 mg/kg in saline) or saline at embryonic days 19.5 and 20.5. The Ara-C and control rats were tested for acoustic startle response and PPI at preadolescent and post-adolescent ages; postnatal day (Pnd) 35 and 56, respectively. Apomorphine (2.0 mg/kg) or phencyclidine (3.0 mg/kg), was given prior to PPI sessions in order to disrupt PPI.
Results
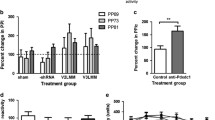
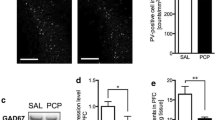
At Pnd 35, Ara-C treatment did not significantly affect acoustic startle amplitudes or PPI. However, at PND 56, Ara-C treated rats had significantly lower acoustic startle amplitudes and significantly diminished sensorimotor gating. Pharmacological challenge with the dopamine agonist apomorphine and the glutamate antagonist PCP significantly disrupted sensorimotor gating in the control subjects. Apomorphine did not further disrupt the existing deficit in the Ara-C treated rats. Ara-C treatment did not cause gross loss of neuronal tissue, although there was a subtle and variable disorganization of the pyramidal cell layer in the hippocampal CA2/3 region.
Conclusion
The results provide evidence to suggest that late embryonic exposure to Ara-C disrupts the circuitry involved in mediating PPI. While the dopamine agonist apomorphine caused a significant disruption in the control rats it did not further disrupt the existing deficit in the Ara-C treated rats. These data provide evidence to support the contention that modest neurodevelopmental insults can significantly affect sensorimotor gating processes in an adult onset dependent manner.











Similar content being viewed by others
References
Abdel-Aziz W, Jiang HY, Hickey RJ, Malkas LH (2000) Ara-C affects formation of cancer cell DNA synthesome replication intermediates. Cancer Chemother Pharmacol 45:312–319
Adlard BP, Dobbing J, Sands J (1975) A comparison of the effects of cytosine arabinoside and adenine arabinoside on some aspects of brain growth and development in the rat. Br J Pharmacol 54:33–39
Arnold SE, Franz BR, Gur RC, Gur RE, Shapiro RM, Moberg PJ, Trojanowski JQ (1995) Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical-hippocampal interactions. Am J Psychiatry 152:738–748
Bayer SA, Altman J, Russo RJ, Zhang X (1993) Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14:83–144
Benes FM, Sorensen I, Bird ED (1991) Reduced neuronal size in posterior hippocampus of schizophrenic patients. Schizophr Bull 17:597–608
Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C (2002) Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology 26:204–215
Braff DL, Geyer MA (1990) Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry 47:181–188
Braff DL, Swerdlow NR, Geyer MA (1999) Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am J Psychiatry 156:596–602
Braff DL, Geyer MA, Swerdlow NR (2001) Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology 156:234–258
Cadenhead KS, Geyer MA, Butler RW, Perry W, Sprock J, Braff DL (1997) Information processing deficits of schizophrenia patients: relationship to clinical ratings, gender and medication status. Schizophr Res 28:51–62
Chen S, Hillman DE (1986) Selective ablation of neurons by methylazoymethanol during pre- and postnatal brain development. Exp Neurol 94:103–119
Christison GW, Casanova MF, Weinberger DR, Rawlings R, Kleinman JE (1989) A quantitative investigation of hippocampal pyramidal cell size, shape, and variability of orientation in schizophrenia. Arch Gen Psychiatry 46:1027–1032
Courtney MJ, Coffey ET (1999) The mechanism of Ara-C-induced apoptosis of differentiating cerebellar granule neurons. Eur J Neurosci 11:1073–1084
Dambska M, Haddad R, Kozlowski PB, Lee MH, Shek J (1982) Telencephalic cytoarchitectonics in the brains of rats with graded degrees of micrencephaly. Acta Neuropathol 58:203–209
Depatie L, Lal S (2001) Apomorphine and the dopamine hypothesis of schizophrenia: a dilemma? J Psychiatr Neurosci 26:203–220
Duval F, Mokrani MC, Crocq MA, Bailey PE, Diep TS, Correa H, Macher JP (2000) Dopaminergic function and the cortisol response to dexamethasone in psychotic depression. Prog Neuropsychopharmacol Biol Psychiatry 24:207–225
Ellenbroek BA, Lubbers LJ, Cools AR (2002) The role of hippocampal dopamine receptors in prepulse inhibition. Eur J Neurosci 15:1237–1243
Feinberg A, Zedeck MS (1980) Production of a highly reactive alkylating agent from the organospecific carcinogen methylazoxymethanol by alcohol dehydrogenase. Cancer Res 40:4446–4450
Feldon J, Weiner I (1988) Long-term attentional deficit in nonhandled males: possible involvement of the dopaminergic system. Psychopharmacology 95:231–236
Ferguson SA, Holson RR, Paule MG (1994) Effects of methylazoxymethanol-induced micrencephaly on temporal response diffferentiation and progressive ration responding in rats. Behav Neural Biol 62:77–81
Ferguson SA, Paule MG, Holson RR (1996) Functional effects of methylazoxymethanol-induced cerebellar hypoplasia in rats. Neurotoxicol Teratol 18:529–537
Fiala E, Stathopoulos C (1984) Metabolism of methylazoxymethanol acetate in the F344 rat and strain-2 guinea pig and its inhibition by pyrazole and disulfiram. J Cancer Res Clin Oncol 108:129–134
Fiore M, Talamini L, Angelucci F, Koch T, Aloe L, Korf J (1999) Prenatal methylazoxymethanol acetate alters behavior and brain NGF levels in young rats: a possible correlation with the development of schizophrenia-like deficits. Neuropharmacology 38:857–869
Fiore M, Korf J, Angelucci F, Talamini L, Aloe L (2000) Prenatal exposure to methylazoxymethanol acetate in the rat alters neurotrophin levels and behavior: considerations for neurodevelopmental diseases [In Process Citation]. Physiol Behav 71:57–67
Fiore M, Aloe L, Westenbroek C, Amendola T, Antonelli A, Korf J (2001) Bromodeoxyuridine and methylazoxymethanol exposure during brain development affects behavior in rats: consideration for a role of nerve growth factor and brain derived neurotrophic factor. Neurosci Lett 309:113–116
Fiore M, Korf J, Antonelli A, Talamini L, Aloe L (2002) Long-lasting effects of prenatal MAM treatment on water maze performance in rats: associations with altered brain development and neurotrophin levels. Neurotoxicol Teratol 24:179–191
Freeman J, Barone S, Stanton M (1995) Disruption of cerebellar maturation by an antimitotic agent impairs the ontogeny of eyeblink conditioning in rats. J Neurosci 15:7301–7314
Geyer MA, Wilkinson LS, Humby T, Robbins TW (1993) Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry 34:361–372
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR (2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 156:117–154
Ghaiarnia M, Moore H, Grace AA (1998) Enhanced behavioral effects of phencyclidine (PCP) in rats with developmental abnormalities of the temporal lobe. Soc Neurosci Abstr 24:2177
Gillies K, Price DJ (1993) The fates of cells in the developing cerebral cortex of normal and methylazoxymethanol acetate-lesioned mice. Eur J Neurosci 5:73–584
Gmeiner WH, Skradis A, Pon RT, Liu J (1998) Cytarabine-induced destabilization of a model Okazaki fragment. Nucleic Acids Res 26:2359–2365
Gray LE Jr, Kavlok RJ, Ostby J, Ferrell J, Rogers J, Gray K (1986) An evaluation of figure-eight maze activity and general behavioral development following prenatal exposure to forty chemicals: effects of cytosine arabinoside, dinocap, nitrofen, and vitamin A. Neurotoxicology 7:449–462
Groothuis DR, Benalcazar H, Allen CV, Wise RM, Dills C, Dobrescu C, Rothholtz V, Levy RM (2000) Comparison of cytosine arabinoside delivery to rat brain by intravenous, intrathecal, intraventricular and intraparenchymal routes of administration. Brain Res 856:281–290
Harrison PJ (1999a) The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain 122:593–624
Harrison PJ (1999b) The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain 122:593–624
Johnston MV, Coyle JT (1979) Histological and neurochemical effects of fetal treatment with methylazoxymethanol on rat neocortex in adulthood. Brain Res 170:135–155
Jonsson SA, Luts A, Guldberg-Kjaer N, Brun A (1997) Hippocampal pyramidal cell disarray correlates negatively to cell number: implications for the pathogenesis of schizophrenia. Eur Arch Psychiatry Clin Neurosci 247:120–127
Jonsson SA, Luts A, Guldberg-Kjaer N, Ohman R (1999) Pyramidal neuron size in the hippocampus of schizophrenics correlates with total cell count and degree of cell disarray. Eur Arch Psychiatr Clin Neurosci 249:169–173
Koenig JI, Kirkpatrick B, Lee P (2002) Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology 27:309–318
Kovelman JA, Scheibel AB (1984) A neurohistological correlate of schizophrenia. Biol Psychiatry 19:1601–1621
Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, McCance E, Rosenblatt W, Fingado C, Zoghbi SS, Baldwin RM, Seibyl JP, Krystal JH, Charney DS, Innis RB (1996) Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci USA 93:9235–9240
Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R (1999) Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 46:56–72
Matsutani T, Tamaru M, Hayakawa Y, Nagayoshi M, Nakahara T, Tsukada Y (1983) A neurochemical study of developmental impairment of the brain caused by the administration of cytosine arabinoside during the fetal or neonatal period of rats. Neurochem Res 8:1295–1306
Meltzer HY, Lee MA, Jayathilake K (2001) The blunted plasma cortisol response to apomorphine and its relationship to treatment response in patients with schizophrenia. Neuropsychopharmacology 24:278–290
Mohn AR, Gainetdinov RR, Caron MG, Koller BH (1999) Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 98:427–436
Moore H, Grace AA (1997) Anatomical changes in limbic structures produced by MAM during brain development are associated with changes in physiological interactions among afferents to the nucleus accumbens. Soc Neurosci Abstr 23:2378
Moore H, Giracello D, Grace AA, Geyer MA (1999) Sensory gating deficits in rats with early disruption of limbic cortical development: relevance to schizophrenia. Soc Neurosci Abstr 25:1580
Ohno M (1984) Neuroanatomical study of somatomotor cortex in microcephalic mice induced by cytosine arabinoside. Brain Dev 6:528–538
Ono K, Tokunaga A, Mizukawa K, Kurose K, Tanaka H (1992) Abnormal expression of embryonic neural cell adhesion molecule (N-cam) in the developing mouse cerebellum after neonatal administration of cytosine arabinoside. Dev Brain Res 65:119–122
Percy DH (1975) Taratogenic effects of the pyrimidine analogues 5-iododeoxyuridine and cytosine arabinoside in late fetal mice and rats. Teratology 11:103–117
Perry W, Braff DL (1994) Information-processing deficits and thought disorder in schizophrenia. Am J Psychiatry 151:363–367
Perry W, Geyer MA, Braff DL (1999) Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients. Arch Gen Psychiatry 56:277–281
Sarraf CE, Ansari TW, Conway P, Notay M, Hill S, Alison MR (1993) Bromodeoxyuridine-labelled apoptosis after treatment with antimetabolites in two murine tumours and in small intestinal crypts. Br J Cancer 68:678–680
Scheibel AB, Kovelman JA (1981) Disorientation of the hippocampal pyramidal cell and its processes in the schizophrenia patients. Biol Psychiatry 16:101–102
Swerdlow NR, Geyer MA (1998) Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophr Bull 24:285–301
Swerdlow NR, Geyer MA (1999) Neurophysiology and neuropharmacology of short lead interval startle modification. In: Dawson ME, Schell AM, Bohmelt AH (eds) Startle modification: implications for neuroscience, cognitive science and clinical science. Cambridge University Press, Cambridge, pp 114–133
Swerdlow NR, Caine SB, Geyer MA (1991) Opiate-dopamine interactions in the neural substrates of acoustic startle gating in the rat. Prog Neuropsychopharmacol Biol Psychiatry 15:415–426
Swerdlow NR, Braff DL, Taaid N, Geyer MA (1994) Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry 51:139–54
Swerdlow NR, Braff DL, Geyer MA (2000a) Animal models of deficient sensorimotor gating: what we know, what we think we know, and what we hope to know soon. Behav Pharmacol 11:185–204
Swerdlow NR, Taaid N, Halim N, Randolph E, Kim YK, Auerbach P (2000b) Hippocampal lesions enhance startle gating-disruptive effects of apomorphine in rats: a parametric assessment. Neuroscience 96:523–536
Swerdlow NR, Geyer MA, Braff DL (2001) Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology 156:194–215
Talamini LM, Koch T, Ter Horst GJ, Korf J (1998) Methylazoxymethanol acetate-induced abnormalities in the entorhinal cortex of the rat; parallels with morphological findings in schizophrenia. Brain Res 789:293–306
Talamini LM, Koch T, Luiten PG, Koolhaas JM, Korf J (1999) Interruptions of early cortical development affect limbic association areas and social behaviour in rats; possible relevance for neurodevelopmental disorders. Brain Res 847:105–120
Talamini LM, Ellenbroek B, Koch T, Korf J (2000) Impaired sensory gating and attention in rats with developmental abnormalities of the mesocortex. Implications for schizophrenia. Ann N Y Acad Sci 911:486–494
Tamminga CA, Schaffer MH, Smith RC, Davis JM (1978) Schizophrenic symptoms improve with apomorphine. Science 200:567–568
Tsuang MT, Stone WS, Faraone SV (2001) Genes, environment and schizophrenia. Br J Psychiatry 178: S18–24
Weinberger DR (1996) On the plausibility of “The neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology 14:1s–11s
Weiss IC, Feldon J (2001) Environmental animal models for sensorimotor gating deficiencies in schizophrenia: a review. Psychopharmacology 156:305–326
Woodward DJ, Bickett D, Chanda R (1975) Purkinje cell dendritic alterations after transient developmental injury of the external granular layer. Brain Res 97:195–214
Zhang W-N, Bast T, Feldon J (2002) Effects of hippocampal N-methly-d-aspartate infusion on locomotor activity and prepulse inhibition: differences between the dorsal and ventral hippocampus. Behav Neurosci 116:72–84
Acknowledgements
This study was supported by a University-Industry partnership with Novartis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elmer, G.I., Sydnor, J., Guard, H. et al. Altered prepulse inhibition in rats treated prenatally with the antimitotic Ara-C: an animal model for sensorimotor gating deficits in schizophrenia. Psychopharmacology 174, 177–189 (2004). https://doi.org/10.1007/s00213-003-1757-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1757-7