Abstract
Rationale
It has been reported that human opiate addicts discount delayed rewards more than non-addicts, indicating that they are more impulsive. However, it is not clear whether this difference reflects pre-existing traits, or the effects of exposure to the opiates.
Objectives
This study was designed to investigate the effects of an opioid agonist and antagonist on delay discounting in rats. The study had three objectives: to determine (1) the acute effects of the opioid agonist morphine (MOR) on delay discounting, (2) the acute effects of the opioid antagonist naltrexone (NAL) on delay discounting, and (3) whether NAL reverses the effects of MOR on delay discounting.
Methods
An adjusting amount procedure (AdjAmt) was used to determine how much animals discounted the value of delayed rewards. Acute doses of MOR (0.3, 1.0, and 1.8 mg/kg SC), NAL (0.01, 0.1, 1.0, and 10 mg/kg SC) and NAL (0.1 mg/kg SC) prior to MOR (1.8 mg/kg SC) were tested in 15 rats.
Results
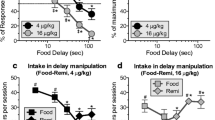
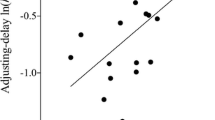
MOR dose dependently increased the rate of delay discounting (i.e., made the animals more impulsive). NAL alone had no effect on the value of delayed rewards, but NAL blocked the effects of MOR.
Conclusions
These results suggested that the direct effects of MOR may contribute to the high level of impulsive behavior seen among opiate users.



Similar content being viewed by others
References
Ainslie G (1975) Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull 82:463–496
Berridge KC (1996) Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 20:1–25
Brown DR, Holtzman SG (1979) Suppression of deprivation-induced food and water intake in rats and mice by naloxone. Pharmacol Biochem Behav 11:567–573
Brown DR, Blank MS, Holtzman SG (1980) Suppression by naloxone of water intake induced by deprivation and hypertonic saline in intact and hypophysectomized rats. Life Sci 26:1535–1542
Cardinal RN, Robbins TW, Everitt BJ (2000) The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology 152:362–375
Castellano C, Puglisi-Allegra S (1982) Effects of naloxone and naltrexone on locomotor activity in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav 16:561–563
De Oliveira RW, Nakamura-Palacios EM (2003) Haloperidol increases the disruptive effect of alcohol on spatial working memory in rats: a dopaminergic modulation in the medial prefrontal cortex. Psychopharmacology 170:51–61
Di Chiara G, Imperato A (1988) Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 244:1067–1080
Evenden JL, Ryan CN (1996) The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology 128:161–170
Finn PR, Justus A, Mazas C, Steinmetz JE (1999) Working memory, executive processes and the effects of alcohol on go/no-go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology 146:465–472
Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ (2002) Mild opiate deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology 163:174–182
Grace RC (1999) The matching law and amount-dependent exponential discounting as accounts of self-control choice. J Exp Anal Behav 71:27–44
Green L, Myerson J, McFadden E (1997) Rate of temporal discounting decreases with amount of reward. Mem Cognit 25:715–723
Herrnstein RJ (1981) Self-control as response strength. In: Bradshaw CM, Szabadi E, Lowe CF (eds) Quantification of steady-state operant behavior. Elsevier, Amsterdam, pp 3–20
Hinson JM, Jameson TL, Whitney P (2003) Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn 29:298–306
Itoh J, Ukai M, Kameyama T (1994) Dynorphin A-(1–13) potently improves the impairment of spontaneous alternation performance induced by the mu-selective opioid receptor agonist DAMGO in mice. J Pharmacol Exp Ther 269:15–21
Iwamoto ET (1984) An assessment of the spontaneous activity of rats administered morphine, phencyclidine, or nicotine using automated and observational methods. Psychopharmacology 84:374–382
Johnson MW, Bickel WK (2002) Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav 77:129–146
Kirby KN, Petry NM, Bickel WK (1999) Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen 128:78–87
Koek W, Slangen JL (1984) Acute effects of naloxone and naltrexone, but lack of delayed effects, on exploratory behavior in the rat. Psychopharmacology 84:383–387
Kuzmin A, Sandin J, Terenius L, Ogren SO (2000) Dose- and time-dependent bimodal effects of kappa-opioid agonists on locomotor activity in mice. J Pharmacol Exp Ther 295:1031–1042
Logue AW (1988) Research on self-control: an integrated framework. Behav Brain Sci 11:665–709
Luce RD (1986) Response times: their role in inferring elementary mental organization. Oxford University Press, New York
Madden GJ, Petry NM, Badger GJ, Bickel WK (1997) Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol 5:256–262
Madden GJ, Bickel WK, Jacobs EA (1999) Discounting of delayed rewards in opioid-dependent outpatients: exponential or hyperbolic discounting functions? Exp Clin Psychopharmacol 7:284–293
Martin WR (1967) Opioid antagonists. Pharmacol Rev 19:463–521
McMillan DE (1973) Effects of narcotics and narcotic antagonists on operant behavior. Adv Biochem Psychopharmacol 8:345–359
Molinengo L (1964) Effects of morphine on the operant behaviour in rats. Psychopharmacologia 6:347–367
Oas P (1985) The psychological asses2ment of impulsivity: a reveiw. J Psychoedu Assess 3:141–156
Odum AL, Schaal DW (2000) The effects of morphine on fixed-interval patterning and temporal discrimination. J Exp Anal Behav 74:229–243
Odum AL, Madden GJ, Badger GJ, Bickel WK (2000) Needle sharing in opioid-dependent outpatients: psychological processes underlying risk. Drug Alcohol Depend 60:259–266
Pan ZZ (1998) mu-Opposing actions of the kappa-opioid receptor. Trends Pharmacol Sci 19:94–98
Petry NM (2001) Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology 154:243–250
Powell KJ, Abul-Husn NS, Jhamandas A, Olmstead MC, Beninger RJ, Jhamandas K (2002) Paradoxical effects of the opioid antagonist naltrexone on morphine analgesia, tolerance, and reward in rats. J Pharmacol Exp Ther 300:588–596
Rachlin H (1989) Judgment, decision, and choice: a cognitive/behavioral synthesis. W.H. Freeman and Company, New York
Rachlin H, Green L (1972) Commitment, choice and self-control. J Exp Anal Behav 17:15–22
Rachlin H, Raineri A, Cross D (1991) Subjective probability and delay. J Exp Anal Behav 55:233–244
Rada P, Mark GP, Pothos E, Hoebel BG (1991) Systemic morphine simultaneously decreases extracellular acetylcholine and increases dopamine in the nucleus accumbens of freely moving rats. Neuropharmacology 30:1133–1136
Richards JB, Mitchell SH, de Wit H, Seiden LS (1997) Determination of discount functions in rats with an adjusting-amount procedure. J Exp Anal Behav 67:353–366
Richards JB, Sabol KE, de Wit H (1999a) Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology (Berl) 146:432–9
Richards JB, Zhang L, Mitchell SH, de Wit H (1999b) Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav 71:121–143
Sawaguchi T (2000) The role of D1-dopamine receptors in working memory-guided movements mediated by frontal cortical areas. 7:9–19
Sawaguchi T, Goldman-Rakic PS (1991) D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 251:947–950
Sawaguchi T, Goldman-Rakic PS (1994) The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol 71:515–528
Silva MT, Heyman GM (2001) Chronic morphine consumption decreases wheel running and wheel running-reinforced behavior in rats. Pharmacol Biochem Behav 69:51–57
Uhl GR, Childers S, Pasternak G (1994) An opiate-receptor gene family reunion. Trends Neurosci 17:89–93
Vasko MR, Domino EF (1978) Tolerance development to the biphasic effects of morphine on locomotor activity and brain acetylcholine in the rat. J Pharmacol Exp Ther 207:848–858
Wade TR, de Wit H, Richards JB (2000) Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology 150:90–101
White DA, Holtzman SG (2001) Acute opioid pretreatment potentiates naltrexone-induced drinking suppression in water-deprived rats. J Pharmacol Exp Ther 298:156–164
Wise RA, Leone P, Rivest R, Leeb K (1995) Elevations of nucleus accumbens dopamine and DOPAC levels during intravenous heroin self-administration. Synapse 21:140–148
Acknowledgements
We thank Mark Kogutowski for technical assistance and Dr. Jerrold Winter for his helpful advice. The National Institute on Drug Abuse (DA-10588) supported this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kieres, A.K., Hausknecht, K.A., Farrar, A.M. et al. Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology 173, 167–174 (2004). https://doi.org/10.1007/s00213-003-1697-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1697-2