Abstract
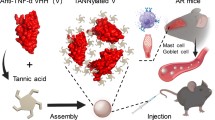
Curcumin (CUR) has been considered a potential therapeutic agent for allergic reactions due to its antioxidant and anti-inflammatory activities. Nanofibers have attracted increasing attention in drug delivery. The aim of this study was to investigate the combined therapeutic effects of curcumin and allergen in nanofiber-based treatments in order to increase the effectiveness of sublingual immunotherapy (SLIT) efficacy in a mouse model of allergic rhinitis. Nanofibers containing CUR (1.25% and 2.5%) and ovalbumin 2% (OVA) as an allergen were prepared via electrospinning and characterized. BALB/c mice were sensitized with OVA to the induced allergic rhinitis model. SLIT with free and/or nanofibers was carried out. IL-4, INF-γ, and IgE serum levels were measured using ELISA. Splenocyte proliferation was evaluated by the MTT assay. Lung and nasal histological examinations and nasal lavage fluid (NALF) cell counting were carried out. Nanofibers containing 1.25% CUR and 2% OVA were chosen as the optimal formulations. SLIT treatment with the CUR and OVA nanofiber co-administration led to a significantly decreased serum IgE. Nanofiber containing 2.5 µg of CUR/mouse combined with OVA nanofiber showed a significant decrease in IL-4 and an increase in IFN-γ compared to other groups. NALF assessment showed a significant decrease in specific cell and eosinophil counts in the treated nanofiber groups. The histopathological results of NAL in the optimal formulations were near normal, with diminished cellular infiltration and inflammation. Our findings suggest that co-sublingual administration of allergen and CUR nanofibers can be considered as potential immunomodulatory agents.












Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study.
Abbreviations
- AR:
-
Allergic rhinitis
- AIT:
-
Allergen immunotherapy
- SLIT:
-
Sublingual immunotherapy
- SCIT:
-
Subcutaneous immunotherapy
- CUR:
-
Curcumin
- OVA:
-
Ovalbumin
- PVP K90:
-
Povidone polyvinylpyrrolidone K90
- IL-4:
-
Interleukin-4
- IFN-γ:
-
Interferon gamma
- IgE:
-
Immunoglobulin E
- NALF:
-
Nasal lavage fluid
- NAL:
-
Nasal lavage
- ELISA:
-
Enzyme-linked immunosorbent assay
- FTIR:
-
Fourier-transform infrared
- SEM:
-
Scanning electron microscope
- DSC:
-
Differential scanning calorimetry
- PBS:
-
Phosphate-buffered saline
References
Adams KE, James MQ (2022) Allergen immunotherapy. Absolute allergy and immunology board review. Springer
Akdis CA, Akdis M (2015) Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. World Allergy Organ J 8:1–12
Ataei M, Roufogalis BD, Majeed M, Shah MA, Sahebkar A (2023) Curcumin nanofibers: a novel approach to enhance the anticancer potential and bioavailability of curcuminoids. Cur Med Chem 30(3):286–303. https://doi.org/10.2174/0929867329666220322110348
Ataei M, Garekani HA, Sani MA, McClements JD, Sadeghi F (2024) Evaluation of polyvinyl pyrrolidone nanofibers for encapsulation, protection, and release of curcumin: impact on in vitro bioavailability. J Mol Liq 397:124115
Baba SM, Rasool R, Gull A, Qureshi TA, Beigh AH, Qadri Q, Shah ZA (2021) Effectiveness of sublingual immunotherapy in the treatment of HDM-induced nasobronchial allergies: a 3-year randomized case-control study from Kashmir. Front Immunol 12:723814
Blaiss MS (2022) Pediatric sublingual allergen immunotherapy. In: Allergy & Asthma Proc 43(4)
Braido F, Sclifò F, Ferrando M, Giorgio Walter C (2014) New therapies for allergic rhinitis. Curr Allergy Asthma Rep 14:1–8
Calderón MoisésA, Frankland AW, Demoly P (2013) Allergen immunotherapy and allergic rhinitis: false beliefs. BMC Med 11:1–5
Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, Bousquet J, Calderón M, Compalati E, Stephen RD (2014) Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J 7:6
Chai R, Liu B, Qi F (2017) The significance of the levels of IL-4, IL-31 and TLSP in patients with asthma and/or rhinitis. Immunotherapy 9:331–37
Chen H-M, Deng-Guang Y (2010) An elevated temperature electrospinning process for preparing acyclovir-loaded PAN ultrafine fibers. J Mater Process Technol 210:1551–1555
Chen Y, Chen YHH, Li X, Qian P (2020) A ferritin nanoparticle vaccine for foot-and-mouth disease virus elicited partial protection in mice. Vaccine 38:5647–5652
Del Giudice MM, Licari A, Brambilla I, Tosca MA, Ciprandi G (2020) Allergen immunotherapy in pediatric asthma: a pragmatic point of view. Child 7(6):58
Dispenza MC (2019) November. Classification of hypersensitivity reactions. In: Aller Asthma Proc 40(6)
Dorofeeva Y, Shilovskiy I, Tulaeva I, Focke-Tejkl M, Flicker S, Kudlay D, Khaitov M, Karsonova A, Riabova K, Karaulov A (2021) Past, present, and future of allergen immunotherapy vaccines. Allergy 76:131–49
Eren Böncü T, Ozdemir N (2022) Effects of drug concentration and PLGA addition on the properties of electrospun ampicillin trihydrate-loaded PLA nanofibers. Beilstein J Nanotechnol 13:245–254
Ghosal K, Augustine R, Zaszczynska A, Barman M, Jain A, Hasan A, Kalarikkal N, Sajkiewicz P, Thomas S (2021) Novel drug delivery systems based on triaxial electrospinning based nanofibers. Reactive Funct Polym 163:104895
Gutowska-Ślesik J, Samoliński B, Krzych-Fałta E (2023) The increase in allergic conditions based on a review of literature. Postepy Dermatol Alergol 40:1–7
Hajavi J, Hashemi M, Sankian M (2019) Evaluation of size and dose effects of rChe a 3 allergen loaded PLGA nanoparticles on modulation of Th2 immune responses by sublingual immunotherapy in mouse model of rhinitis allergic. Int J Pharm 563:282–292
Jo M, Lee J, Kim HG, Kim JK, Kim H, Shin KK, Bach TT, Eum SM, Lee JS, Choung ES (2021) Anti-inflammatory effect of Barringtonia angusta methanol extract is mediated by targeting of Src in the NF-κB signalling pathway. Pharm Biol 59:797–808
Kakli HA, Timothy DR (2016) Allergic rhinitis. Prim Care: Clin Off Pract 43:465–75
Kamranpour S, Mirzaeei S, Daneshgar F, Najafi F (2021) Fast-dissolving sublingual nanofibers of ondansetron hydrochloride: formulation, physicochemical characterization, and clinical evaluation on post-cataract surgery patients. Pharm Sci 28:101–111
Kappen JH, Durham SR, Veen HI, Shamji MH (2017) Applications and mechanisms of immunotherapy in allergic rhinitis and asthma. Ther Adv Respir Dis 11:73–86
Koushki K, Varasteh A-R, Shahbaz SK, Sadeghi M, Mashayekhi K, Ayati SH, Moghadam M, Sankian M (2020) Dc-specific aptamer decorated gold nanoparticles: a new attractive insight into the nanocarriers for allergy epicutaneous immunotherapy. Int J Pharm 584:119403
Lin E-R, Cheng Y-H, Hsiao FS-H, Witold S, Proskura A, Dybus, Yu-Hsiang Y (2019) Optimization of solid-state fermentation conditions of Bacillus licheniformis and its effects on Clostridium perfringens-induced necrotic enteritis in broilers. Rev Bras Zootec 48
Manatunga DC, Jayasinghe JAB, Sandaruwan C, De Silva RM, De Silva KMN (2022) Enhancement of release and solubility of curcumin from electrospun PEO-EC-PVP tripolymer-based nanofibers: a study on the effect of hydrogenated castor oil. ACS Omega 7:37264–37278
McLeod JJA, Baker B, John JR (2015) Mast cell production and response to IL-4 and IL-13. Cytokine 75:57–61
Moon D-O (2024) Curcumin in cancer and inflammation: an in-depth exploration of molecular interactions, therapeutic potentials, and the role in disease management. Int J Mol Sci 25:2911
Nakagome K, Fujio K, Nagata M (2023) Potential effects of AIT on nonspecific allergic immune responses or symptoms. J Clin Med 12:3776
Nezarati RM, Eifert MB, Cosgriff-Hernandez E (2013) Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng Part C: Methods 19(10):810-819. https://doi.org/10.1089/ten.tec.2012.0671
Pant B, Park M, Soo-Jin P (2019) Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: a review. Pharmaceutics 11:305
Plum T, Binzberger R, Thiele R, Shang F, Postrach D, Fung C, Fortea M, Stakenborg N, Wang Z, Tappe-Theodor A, Poth T, MacLaren DAA, Boeckxstaens G, Kuner R, Pitzer C, Monyer H, Xin C, Bonventre JV, Tanaka S, Voehringer D, Vanden Berghe P, Strid J, Feyerabend TB, Rodewald HR (2023) Mast cells link immune sensing to antigen-avoidance behaviour. Nature 620:634–642
Ren Jian-jun YuZ, Yang F-L, Lv D, Hung S, Zhang J, Lin P, Liu S-X, Zhang N, Bachert C (2015) Effects of Bifidobacterium breve feeding strategy and delivery modes on experimental allergic rhinitis mice. PLoS ONE 10:e0140018
Shahbaz S, Keshavarz A-R, Varasteh K, Koushki SH, Ayati K, Mashayekhi M, Sadeghi M, Sankian M, Moghadam M (2020) Sublingual dendritic cells targeting by aptamer: possible approach for improvement of sublingual immunotherapy efficacy. Int Immunopharmacol 85:106603
Shahgordi S, Sankian M, Yazdani Y, Mashayekhi K, Ayati SH, Sadeghi M, Saeidi M, Hashemi M (2020) Immune responses modulation by curcumin and allergen encapsulated into PLGA nanoparticles in mice model of rhinitis allergic through sublingual immunotherapy. Int Immunopharmacol 84:106525
Shan X, Liu C, Li F, Ouyang C, Gao Q, Zheng K (2015) Nanoparticles vs. nanofibers: a comparison of two drug delivery systems on assessing drug release performance in vitro Designed Monomers and Polymers 18(7):678–689. https://doi.org/10.1080/15685551.2015.1070500
Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka C, Zgheib R, Zam W, Sestito S, Rapposelli S, Zielińska D, William NS (2020) Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front Pharmacol 11:550909
Shi Z, Jiang W, Wang M, Wang X, Li X, Chen X, Li Q (2017) Inhibition of JAK/STAT pathway restrains TSLP-activated dendritic cells mediated inflammatory T helper type 2 cell response in allergic rhinitis. Mol cell Biochem 430:161–69
Sofi HS, Abdal-hay A, Ivanovski S, Zhang YS, Sheikh FA (2020) Electrospun nanofibers for the delivery of active drugs through nasal, oral and vaginal mucosa: current status and future perspectives. Mater Sci Eng: C 111:110756
Terada T (2022) Alternatives to subcutaneous immunotherapy for allergic rhinitis. Allergies 2:23–32
Thakare VN, Osama MM, Suresh RN (2013) Therapeutic potential of curcumin in experimentally induced allergic rhinitis in guinea pigs. Int Immunopharmacol 17:18–25
Thenmozhi S, Dharmaraj N, Kadirvelu K, Kim HY (2017) Electrospun nanofibers: new generation materials for advanced applications. Mater Sci Eng: B 217:36–48
Tsabouri S, Mavroudi A, Feketea G, George VG (2017) Subcutaneous and sublingual immunotherapy in allergic asthma in children. Front Pead 5:82
Virchow JC, Pfaar O, Lommatzsch M (2024) Allergen immunotherapy for allergic asthma. Allergol Select 8:6–11
Vitaliti G, Leonardi S, Miraglia Del Giudice M, Salpietro A, Artusio L, Caimmi D, Arrigo T, Salpietro C, Ciprandi G, La Rosa M (2012) Mucosal immunity and sublingual immunotherapy in respiratory disorders. J Biol Regul Homeost Agents 26:S85-93
Wang C, Ma C, Wu Z, Liang H, Yan P, Song J, Ma N, Zhao Q (2015) Enhanced bioavailability and anticancer effect of curcumin-loaded electrospun nanofiber: in vitro and in vivo study. Nanoscale Res Lett 10:1–10
Wang M, Gu Z, Yang J, Zhao H, and Zhiwei Cao (2019) Changes among TGF-β1 + Breg cells and helper T cell subsets in a murine model of allergic rhinitis with prolonged OVA challenge. Int Immunopharmacol 69:347–357. https://doi.org/10.1016/j.intimp.2019.01.009
Wani M, Ummer P, Kaur, Kaur P (2019) Nanofiber for sublingual delivery: a review. J Bio-Pharma Res 8:2781–2787
Wheatley LM, Togias A (2015) Allergic rhinitis. N Engl J Med 372:456–463
Witika BA, Makoni PA, Matafwali SK, Mweetwa LL, Shandele GC, Walker RB (2021) Enhancement of biological and pharmacological properties of an encapsulated polyphenol: curcumin. Molecules 26:4244
Wong S-C, Baji A, Leng S (2008) Effect of fiber diameter on tensile properties of electrospun poly(ɛ-caprolactone). Polymer 49(21):4713–4722. https://doi.org/10.1016/j.polymer.2008.08.022
Wu S, and Dajiang Xiao (2016) Effect of curcumin on nasal symptoms and airflow in patients with perennial allergic rhinitis. Ann Allergy Asthma Immunol 117:697–702e1
Yang J, and Sihong Lei (2023) Efficacy and safety of sublingual versus subcutaneous immunotherapy in children with allergic rhinitis: a systematic review and meta-analysis. Front Immunol 14:1274241
Yuan H, Li B, Liang K, Lou X, Zhang Y (2014) Regulating drug release from pH-and temperature-responsive electrospun CTS-g-PNIPAAm/poly (ethylene oxide) hydrogel nanofibers. Biomed Mater 9:055001
Zhang N, Li H, Jia J, He M (2015) Anti-inflammatory effect of curcumin on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse. Cell Immunol 298:88–95
Zhu Y, Yu J, Zhu XH, Yuan JS, Dai MN, Bao YW, Jiang Y (2023) Experimental observation of the effect of immunotherapy on CD4 + T cells and Th1/Th2 cytokines in mice with allergic rhinitis. Sci Rep 13:5273
Zong X, Kim K, Fang D, Ran S, Hsiao BS, Chu B (2002) Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 43:4403–4412
Funding
This work was supported by the deputy of research at Mashhad University of Medical Sciences, Mashhad, Iran, under Grant No. (981631).
Author information
Authors and Affiliations
Contributions
M.H. and M.S. designed the study. B.A. and A.S. carried out the experiment with the participation of other authors. B.A. processed the data, performed the analysis, and wrote the manuscript with support from M.H. and M.S. M.M. helped supervise the project. All authors discussed the results and commented on the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Ethical approval
All the animal experiments and handling methods performed in this study were approved by the Institutional Animal Ethics Committee of the Mashhad University of Medical Sciences (IR.MUMS.PHARMACY.REC.1399.020).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansari, B., Abbaspour, M.R., Estajy, A. et al. Development of fast-dissolving sublingual nanofibers containing allergen and curcumin for immune response modulation in a mouse model of allergic rhinitis. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03139-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03139-y