Abstract
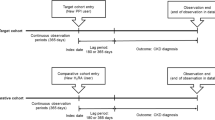
Proton pump inhibitor (PPI) use may be associated with renal dysfunction. Renal dysfunction in PPI users requires evaluation of development and progression risks simultaneously, using estimated glomerular filtration rate (eGFR) slope, which indicates changes in eGFR per year. To the best of our knowledge, no studies have evaluated eGFR slope in PPI users. This study investigated the association between PPI use and renal dysfunction using eGFR slope. A single-center cohort study was conducted using the health records data at Hamamatsu University Hospital in Japan. Participants were defined as first users of acid-suppressing drugs (PPIs or Histamine H2 receptor antagonists (H2RAs)) from 2010 to 2021 and continuously prescribed for ≥ 90 days. The H2RA group was used for the propensity-score matching (PSM) to the PPI group to minimize the effects of confounders. The eGFR slope was estimated using a linear mixed effects model. Participants were stratified by baseline eGFR and age, respectively, as subgroup analyses. A total of 4,649 acid-suppressing drug users met the inclusion criteria, including 950 taking H2RAs and 3,699 PPIs. After PSM, 911 patients were assigned to each group. The eGFR slopes of the PPI and H2RA users were -4.75 (95% CI: -6.29, -3.20) and -3.40 (-4.38, -2.42), respectively. The difference between the groups was not significant. Significant declines in eGFR were observed with PPIs with baseline eGFR ≥ 90 and age < 65. PPI use for ≥ 90 days may hasten eGFR decline compared to H2RA use, especially in patients with eGFR ≥ 90 or age < 65.



Similar content being viewed by others
Data availability
Data is not available due to Hamamatsu University School of Medicine’s legislation. Restrictions apply to the availability of the datasets generated and/or analyzed during the current study, and so they are not publicly available due to confidentiality.
Code availability
We can provide a part of information if requested.
References
Antoniou T, Macdonald EM, Hollands S, Gomes T, Mamdani MM, Garg AX, Paterson JM, Juurlink DN (2015) Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open 3(2):E166–171. https://doi.org/10.9778/cmajo.20140074
Arora P, Gupta A, Golzy M, Patel N, Carter RL, Jalal K, Lohr JW (2016) Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol 17(1):112. https://doi.org/10.1186/s12882-016-0325-4
Austin PC (2011) Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 10(2):150–161. https://doi.org/10.1002/pst.433
Brewster UC, Perazella MA (2007) Acute kidney injury following proton pump inhibitor therapy. Kidney Int 71(6):589–593. https://doi.org/10.1038/sj.ki.5002038
Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Srivali N, Edmonds PJ, Ungprasert P, O'Corragain OA, Korpaisarn S, Erickson SB (2015) Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail 37(7):1237–1241. https://doi.org/10.3109/0886022X.2015.1057800
Cholin L, Ashour T, Mehdi A, Taliercio JJ, Daou R, Arrigain S, Schold JD, Thomas G, Nally J, Nakhoul NL, Nakhoul GN (2021) Proton-pump inhibitor vs. H2-receptor blocker use and overall risk of CKD progression. BMC Nephrol 22(1):264. https://doi.org/10.1186/s12882-021-02449-0
El Chamieh C, Larabi IA, Laville SM, Jacquelinet C, Combe C, Fouque D, Laville M, Frimat L, Pecoits-Filho R, Lange C, Stengel B, Alencar De Pinho N, Alvarez JC, Massy ZA, Liabeuf S, Chronic Kidney Disease-Renal E, Information Network Study G (2023) Proton-pump inhibitors and serum concentrations of uremic toxins in patients with chronic kidney disease. Toxins (Basel) 15(4). https://doi.org/10.3390/toxins15040276
Galmiche JP, Hatlebakk J, Attwood S, Ell C, Fiocca R, Eklund S, Langstrom G, Lind T, Lundell L, Collaborators LT (2011) Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 305(19):1969–1977. https://doi.org/10.1001/jama.2011.626
Geevasinga N, Coleman PL, Webster AC, Roger SD (2006) Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol 4(5):597–604. https://doi.org/10.1016/j.cgh.2005.11.004
Harder VS, Stuart EA, Anthony JC (2010) Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 15(3):234–249. https://doi.org/10.1037/a0019623
Hart E, Dunn TE, Feuerstein S, Jacobs DM (2019) Proton pump inhibitors and risk of acute and chronic kidney disease: a retrospective cohort study. Pharmacotherapy 39(4):443–453. https://doi.org/10.1002/phar.2235
Hatakeyama Y, Horino T, Matsumoto T, Terada Y, Okuhara Y (2021) Long-term continuous use of proton-pump inhibitors is associated with renal function decline in patients without acute kidney injury. Clin Exp Nephrol 25(10):1087–1092. https://doi.org/10.1007/s10157-021-02066-z
Hemmelgarn BR, Zhang J, Manns BJ, Tonelli M, Larsen E, Ghali WA et al (2006) Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int 69(12):2155–2161. https://doi.org/10.1038/sj.ki.5000270
Imai E, Horio M, Yamagata K, Iseki K, Hara S, Ura N, Kiyohara Y, Makino H, Hishida A, Matsuo S (2008) Slower decline of glomerular filtration rate in the Japanese general population: a longitudinal 10-year follow-up study. Hypertens Res 31(3):433–441. https://doi.org/10.1291/hypres.31.433
Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A (2016) Proton pump inhibitors affect the gut microbiome. Gut 65(5):740–748. https://doi.org/10.1136/gutjnl-2015-310376
Inker LA, Heerspink HJL, Tighiouart H, Levey AS, Coresh J, Gansevoort RT, Simon AL, Ying J, Beck GJ, Wanner C, Floege J, Li PK, Perkovic V, Vonesh EF, Greene T (2019) GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol 30(9):1735–1745. https://doi.org/10.1681/ASN.2019010007
Inker LA, Heerspink HJL, Tighiouart H, Chaudhari J, Miao S, Diva U, Mercer A, Appel GB, Donadio JV, Floege J, Li PKT, Maes BD, Locatelli F, Praga M, Schena FP, Levey AS, Greene T (2021) Association of treatment effects on early change in urine protein and treatment effects on GFR slope in IgA nephropathy: an individual participant meta-analysis. Am J Kidney Dis 78(3):340–349 e341. https://doi.org/10.1053/j.ajkd.2021.03.007
Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, Sutter JL, Welter D, Ley RE, Bell JT, Spector TD, Steves CJ (2016) Proton pump inhibitors alter the composition of the gut microbiota. Gut 65(5):749–756. https://doi.org/10.1136/gutjnl-2015-310861
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Kanda E, Usui T, Kashihara N, Iseki C, Iseki K, Nangaku M (2018) Importance of glomerular filtration rate change as surrogate endpoint for the future incidence of end-stage renal disease in general Japanese population: community-based cohort study. Clin Exp Nephrol 22(2):318–327. https://doi.org/10.1007/s10157-017-1463-0
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3:1–150. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf
Klatte DCF, Gasparini A, Xu H, de Deco P, Trevisan M, Johansson ALV, Wettermark B, Arnlov J, Janmaat CJ, Lindholm B, Dekker FW, Coresh J, Grams ME, Carrero JJ (2017) Association between proton pump inhibitor use and risk of progression of chronic kidney disease. Gastroenterology 153(3):702–710. https://doi.org/10.1053/j.gastro.2017.05.046
Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA (2015) Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS ONE 10(6):e0128004. https://doi.org/10.1371/journal.pone.0128004
Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, Grams ME (2016) Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 176(2):238–246. https://doi.org/10.1001/jamainternmed.2015.7193
Lobera T, Navarro B, Del Pozo MD, Gonzalez I, Blasco A, Escudero R, Venturini M, Alarcon E (2009) Nine cases of omeprazole allergy: cross-reactivity between proton pump inhibitors. J Investig Allergol Clin Immunol 19(1):57–60
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53(6):982–992. https://doi.org/10.1053/j.ajkd.2008.12.034
Melsom T, Norvik JV, Enoksen IT, Stefansson V, Mathisen UD, Fuskevag OM, Jenssen TG, Solbu MD, Eriksen BO (2022) Sex differences in age-related loss of kidney function. J Am Soc Nephrol 33(10):1891–1902. https://doi.org/10.1681/ASN.2022030323
Moayyedi P, Eikelboom JW, Bosch J, Connolly SJ, Dyal L, Shestakovska O, Leong D, Anand SS, Stork S, Branch KRH, Bhatt DL, Verhamme PB, O'Donnell M, Maggioni AP, Lonn EM, Piegas LS, Ertl G, Keltai M, Bruns NC, Muehlhofer E, Dagenais GR, Kim JH, Hori M, Steg PG, Hart RG, Diaz R, Alings M, Widimsky P, Avezum A, Probstfield J, Zhu J, Liang Y, Lopez-Jaramillo P, Kakkar AK, Parkhomenko AN, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Vinereanu D, Tonkin AM, Lewis BS, Felix C, Yusoff K, Metsarinne KP, Fox KAA, Yusuf S, Investigators C (2019) Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology 157(3):682–691 e682. https://doi.org/10.1053/j.gastro.2019.05.056
Moledina DG, Perazella MA (2016) Proton pump inhibitors and CKD. J Am Soc Nephrol 27(10):2926–2928. https://doi.org/10.1681/ASN.2016020192
Muriithi AK, Leung N, Valeri AM, Cornell LD, Sethi S, Fidler ME, Nasr SH (2015) Clinical characteristics, causes and outcomes of acute interstitial nephritis in the elderly. Kidney Int 87(4):820–827. https://doi.org/10.1038/ki.2014.294
Nassar Y, Richter S (2018) Proton-pump inhibitor use and fracture risk: an updated systematic review and meta-analysis. J Bone Metab 25(3):141–151 https://doi.org/10.11005/jbm.2018.25.3.141
Nochaiwong S, Ruengorn C, Awiphan R, Koyratkoson K, Chaisai C, Noppakun K, Chongruksut W, Thavorn K (2018) The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant 33(2):331–342. https://doi.org/10.1093/ndt/gfw470
Perez Pimiento AJ, Prieto Lastra L, Rodriguez Cabreros MI, Gonzalez Sanchez LA, Mosquera MR, Cubero AG (2006) Hypersensitivity to lansoprazole and rabeprazole with tolerance to other proton pump inhibitors. J Allergy Clin Immunol 117(3):707–708. https://doi.org/10.1016/j.jaci.2005.11.001
Raghavan R, Shawar S (2017) Mechanisms of drug-induced interstitial nephritis. Adv Chronic Kidney Dis 24(2):64–71. https://doi.org/10.1053/j.ackd.2016.11.004
Rodriguez-Poncelas A, Barcelo MA, Saez M, Coll-de-Tuero G (2018) Duration and dosing of Proton Pump Inhibitors associated with high incidence of chronic kidney disease in population-based cohort. PLoS ONE 13(10):e0204231. https://doi.org/10.1371/journal.pone.0204231
Rossert J (2001) Drug-induced acute interstitial nephritis. Kidney Int 60(2):804–817. https://doi.org/10.1046/j.1523-1755.2001.060002804.x
Ruffenach SJ, Siskind MS, Lien YH (1992) Acute interstitial nephritis due to omeprazole. Am J Med 93(4):472–473. https://doi.org/10.1016/0002-9343(92)90181-a
Strand DS, Kim D, Peura DA (2017) 25 years of proton pump inhibitors: a comprehensive review. Gut Liver 11(1):27–37. https://doi.org/10.5009/gnl15502
Trifan A, Stanciu C, Girleanu I, Stoica OC, Singeap AM, Maxim R, Chiriac SA, Ciobica A, Boiculese L (2017) Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J Gastroenterol 23(35):6500–6515. https://doi.org/10.3748/wjg.v23.i35.6500
Troost JP, Trachtman H, Spino C, Kaskel FJ, Friedman A, Moxey-Mims MM, Fine RN, Gassman JJ, Kopp JB, Walsh L, Wang R, Gipson DS (2021) Proteinuria reduction and kidney survival in focal segmental glomerulosclerosis. Am J Kidney Dis 77(2):216–225. https://doi.org/10.1053/j.ajkd.2020.04.014
Usui J, Yamagata K, Imai E, Okuyama H, Kajiyama H, Kanamori H, Kaneko S, Kono E, Sakai Y, Sakai N, Sakamaki Y, Taniguchi Y, Nakai K, Nishiwaki H, Hirata S, Yamaya H, Tsuruoka S, Terada Y, Yokoyama H, Wada T, Narita I (2016) Clinical practice guideline for drug-induced kidney injury in Japan 2016: digest version. Clin Exp Nephrol 20(6):827–831. https://doi.org/10.1007/s10157-016-1334-0
van der Burgh AC, Rizopoulos D, Ikram MA, Hoorn EJ, Chaker L (2021) Determinants of the evolution of kidney function with age. Kidney Int Rep 6(12):3054–3063. https://doi.org/10.1016/j.ekir.2021.10.006
Wanner C, Heerspink HJL, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, Hantel S, Woerle HJ, Broedl UC, von Eynatten M, Groop PH, Investigators E-RO (2018) Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME Trial. J Am Soc Nephrol 29(11):2755–2769. https://doi.org/10.1681/ASN.2018010103
Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M (2007) Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int 72(5):632–637. https://doi.org/10.1038/sj.ki.5002374
Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z (2016) Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol 27(10):3153–3163. https://doi.org/10.1681/ASN.2015121377
Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z (2017) Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int 91(6):1482–1494. https://doi.org/10.1016/j.kint.2016.12.021
Yang H, Juang SY, Liao KF (2019) Proton pump inhibitors use and risk of chronic kidney disease in diabetic patients. Diabetes Res Clin Pract 147:67–75. https://doi.org/10.1016/j.diabres.2018.11.019
Yokouchi G, Horio T, Matsumoto N, Fukuda K, Yoshimura R, Fujiwara R, Matsuoka Y, Sakamoto Y, Iwashima Y, Oshiro Y, Fujimoto K, Kasayuki N (2022) Renoprotective effect of chronic treatment with sodium-glucose cotransporter 2 inhibitors and its associated factors in Japanese patients with chronic heart failure and diabetes. Int J Cardiol Heart Vasc 43:101152. https://doi.org/10.1016/j.ijcha.2022.101152
Zhang Z, Kim HJ, Lonjon G, Zhu Y, written on behalf of AMEB-DCTCG (2019) Balance diagnostics after propensity score matching. Ann Transl Med 7(1):16. https://doi.org/10.21037/atm.2018.12.10
Acknowledgements
The authors would like to express his sincere gratitude to Dr. Diego Heman Giunta for discussing the method of statistical analysis. The authors have had the editorial support from Stephen McKay.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Conception and design: Takuma Murofushi, Tatsuya Yagi, Daiki Tsuji, Daisuke Furushima, Tomoyuki Fujikura Collection, analysis, and interpretation of the data: Takuma Murofushi, Tatsuya Yagi Statistical analysis: Tatsuya Yagi, Daisuke Furushima, Kunihiko Itoh, Junichi Kawakami Drafting of the article: Takuma Murofushi, Tatsuya Yagi Critical revision of the article for important intellectual content: Takuma Murofushi, Tatsuya Yagi, Daiki Tsuji, Daisuke Furushima, Tomoyuki Fujikura, Kunihiko Itoh, Junichi Kawakami Final approval of the article: Tatsuya Yagi, Junichi Kawakami Agreeing to be accountable and willing to investigate and resolve all questions pertaining to accuracy and integrity of the work: Takuma Murofushi, Tatsuya Yagi, Daiki Tsuji, Daisuke Furushima, Tomoyuki Fujikura, Kunihiko Itoh, Junichi Kawakami. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This research was approved by the Life Science and Medical Research Ethics Committee of Hamamatsu University School of Medicine and the Research Ethics Review Board of University of Shizuoka on December 31, 2021 (Permission number: 21-253). All researchers involved in this study conducted the study in compliance with the relevant rules, such as the "Declaration of Helsinki" and the "Ethical Guidelines for Medical and Biological Research Involving Human Subjects".
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Murofushi, T., Yagi, T., Tsuji, D. et al. Changes in estimated glomerular filtration rate in patients administered proton pump inhibitors: a single-center cohort study. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-023-02890-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-023-02890-y