Abstract
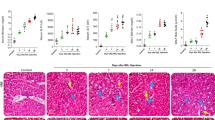
Cholestasis is a clinical complication that primarily influences the liver. However, it is well known that many other organs could be affected by cholestasis. Lung tissue is a major organ influenced during cholestasis. Cholestasis-induced lung injury could induce severe complications such as respiratory distress, serious pulmonary infections, and tissue fibrosis. Unfortunately, there is no specific pharmacological intervention against this complication. Several studies revealed that oxidative stress and inflammatory response play a role in cholestasis-induced lung injury. Carnosine (CARN) is a dipeptide found at high concentrations in different tissues of humans. CARN’s antioxidant and antiinflammatory properties are repeatedly mentioned in various experimental models. This study aimed to assess the role of CARN on cholestasis-induced lung injury. Rats underwent bile duct ligation (BDL) to induce cholestasis. Broncho-alveolar lavage fluid (BALF) levels of inflammatory cells, pro-inflammatory cytokines, and immunoglobulin were monitored at scheduled intervals (7, 14, and 28 days after BDL). Moreover, lung tissue histopathological alterations and biomarkers of oxidative stress were evaluated. A significant increase in BALF inflammatory cells, TNF-α, IL-1β, IL-6, and immunoglobulin-G (IgG) was detected in the BALF of BDL rats. Moreover, lung tissue histopathological changes, collagen deposition, increased TGF-β, and elevated levels of oxidative stress biomarkers were evident in cholestatic animals. It was found that CARN (100 and 500 mg/kg, i.p.) significantly alleviated lung oxidative stress biomarkers, inflammatory response, tissue fibrosis, and histopathological alterations. These data indicate the potential protective properties of CARN in the management of cholestasis-induced pulmonary damage. The effects of CARN on inflammatory response and oxidative stress biomarkers seems to play a crucial role in its protective properties in the lung of cholestatic animals.







Similar content being viewed by others
Data availability
All data generated or analyzed during this investigation are included in this manuscript.
Change history
16 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00210-023-02463-z
References
Abdoli N, Sadeghian I, Azarpira N, Ommati MM, Heidari R (2021) Taurine mitigates bile duct obstruction-associated cholemic nephropathy: effect on oxidative stress and mitochondrial parameters. Clin Exp HEPATOL 7(1):30–40
Ahmad AA, Falla AM, Duffell E, Noori T, Bechini A, Reintjes R, Veldhuijzen IK (2018) Estimating the scale of chronic hepatitis B virus infection among migrants in EU/EEA countries. BMC Infect Dis 18(1):34
Ahmadi N, Ghanbarinejad V, Ommati MM, Jamshidzadeh A, Heidari R (2018) Taurine prevents mitochondrial membrane permeabilization and swelling upon interaction with manganese: implication in the treatment of cirrhosis-associated central nervous system complications. J Biochem Mol Toxicol 32(11):e22216
Ahmadi N, Rezaee Z, Azarpira N, Zahedi S, Saeedi A, Jamshidzadeh A, Heidari R (2021b) A histopathological evaluation on the effect of captopril in cyclophosphamide-induced hemorrhagic cystitis. Trend Pharm Sci 7(1):35–48
Ahmadi A, Niknahad H, Li H, Mobasheri A, Manthari RK, Azarpira N, Mousavi K, Khalvati B, Zhao Y, Sun J, Zong Y, Ommati MM, Heidari R (2021a) The inhibition of NFкB signaling and inflammatory response as a strategy for blunting bile acid-induced hepatic and renal toxicity. Toxicol Lett 349:12-29
Akram J, Reza H, Farzaneh A, Maral R, Forouzan K, Mohammad Mehdi O, Maryam A, Roya F, Arastoo S, Negar A, Asma N (2016) Antimalarial drugs-induced hepatic injury in rats and the protective role of carnosine. Phaem Sci 22(3):170–180
Aydın AF, Küçükgergin C, Özdemirler-Erata G, Koçak-Toker N, Uysal M (2009) The effect of carnosine treatment on prooxidant–antioxidant balance in liver, heart and brain tissues of male aged rats. Biogerontology 11(1):103–109
Bae O-N, Serfozo K, Baek S-H, Lee KY, Dorrance A, Rumbeiha W, Fitzgerald SD, Farooq MU, Naravelta B, Bhatt A, Majid A (2013) Safety and efficacy evaluation of carnosine, an endogenous neuroprotective agent for ischemic stroke. Stroke 44(1):205–212
Boldyrev AA (2012) Carnosine: new concept for the function of an old molecule. Biochemistry (Mosc) 77:313–326
Boldyrev AA, Aldini G, Derave W (2013) Physiology and pathophysiology of carnosine. Physiol Rev 93(4):1803–1845
Boldyrev A, Gallant S, Sukhich G (1999) Carnosine, the protective, anti-aging peptide. Biosci Rep 19:581–587
Budzeń S, Rymaszewska J (2013) The biological role of carnosine and its possible applications in medicine. Adv Clin Exp Med 22(5):739–744
Caruso G, Fresta CG, Musso N, Giambirtone M, Grasso M, Spampinato SF, Merlo S, Drago F, Lazzarino G, Sortino MA, Lunte SM, Caraci F (2019) Carnosine prevents Aβ-induced oxidative stress and inflammation in microglial cells: a key role of TGF-β1. Cells 8(1):64
Chow C-W, Herrera Abreu MT, Suzuki T, Downey GP (2003) Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol 29(4):427–431
Colzani M, Garzon D, Aldini G (2015) Carnosine and derivatives as inhibitors of protein covalent modifications induced by reactive carbonyl species. Imidazole Dipeptides, pp 139–169
Crush KG (1970) Carnosine and related substances in animal tissues. Comp Biochem Physiol 34(1):3–30
Cuzzocrea S, Genovese T, Failla M, Vecchio G, Fruciano M, Mazzon E, Di Paola R, Muià C, La Rosa C, Crimi N, Rizzarelli E, Vancheri C (2007a) Protective effect of orally administered carnosine on bleomycin-induced lung injury. Am J Physiol 292(5):L1095–L1104
Cuzzocrea S, Genovese T, Failla M, Vecchio G, Fruciano M, Mazzon E, Di Paola R, Muià C, La Rosa C, Crimi N, Rizzarelli E, Vancheri C (2007b) Protective effect of orally administered carnosine on bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol 292(5):L1095-1104
Daubeuf F, Frossard N (2014) Eosinophils and the ovalbumin mouse model of asthma. Methods Mol Biol 1178:283–293
De Luca D, Minucci A, Zecca E, Piastra M, Pietrini D, Carnielli VP, Zuppi C, Tridente A, Conti G, Capoluongo ED (2009) Bile acids cause secretory phospholipase A2 activity enhancement, revertible by exogenous surfactant administration. Intensive Care Med 35(2):321–326
Dursun N, Taşkın E, Öztürk F (2011) Protection against adriamycin-induced cardiomyopathy by carnosine in rats: role of endogenous antioxidants. Biol Trace Elem Res 143(1):412–424
Fickert P, Rosenkranz AR (2020) Cholemic nephropathy reloaded. Semin Liver Dis 40(1):91–100
Fouad AA, El-Rehany MA-A, Maghraby HK (2007) The hepatoprotective effect of carnosine against ischemia/reperfusion liver injury in rats. Eur J Pharmacol 572(1):61–68
Fouad AA, Morsy MA, Gomaa W (2008) Protective effect of carnosine against cisplatin-induced nephrotoxicity in mice. Environ Toxicol Pharmacol 25(3):292–297
Fouad AA, Qureshi HA, Yacoubi MT, Al-Melhim WN (2009) Protective role of carnosine in mice with cadmium-induced acute hepatotoxicity. Food Chem Toxicol 47(11):2863–2870
Fu H, Katsumura Y, Lin M, Muroya Y, Hata K, Fujii K, Yokoya A, Hatano Y (2009) Free radical scavenging and radioprotective effects of carnosine and anserine. Radiat Phys Chem 78(12):1192–1197
Ghanbarinejad V, Ahmadi A, Niknahad H, Ommati MM, Heidari R (2019) Carnosine mitigates manganese mitotoxicity in an in vitro model of isolate brain mitochondria. Adv Pharm Bull 9(2):294–301
Ghanbarinejad V, Jamshidzadeh A, Khalvati B, Farshad O, Li H, Shi X, Chen Y, Ommati MM, Heidari R (2021a) Apoptosis-inducing factor plays a role in the pathogenesis of hepatic and renal injury during cholestasis. Naunyn-Schmiedeberg’s Arch Pharmacol 394(6):1191–1203
Ghanbarinejad V, Ommati MM, Jia Z, Farshad O, Jamshidzadeh A, Heidari R (2021b) Disturbed mitochondrial redox state and tissue energy charge in cholestasis. J Biochem Mol Toxicol 35(9):e22846
Gill SS, Suri SS, Janardhan KS, Caldwell S, Duke T, Singh B (2008) Role of pulmonary intravascular macrophages in endotoxin-induced lung inflammation and mortality in a rat model. Respir Res 9(1):69
Guiotto A, Calderan A, Ruzza P, Borin G (2005) Carnosine and carnosine-related antioxidants: a review. Curr Med Chem 12(20):2293–2315
Guney Y, Turkcu UO, Hicsonmez A, Andrieu MN, Guney HZ, Bilgihan A, Kurtman C (2006) Carnosine may reduce lung injury caused by radiation therapy. Med Hypotheses 66(5):957–959
Hamza RZ, El-Shenawy NS (2017) Anti-inflammatory and antioxidant role of resveratrol on nicotine-induced lung changes in male rats. Toxicol Rep 43:99–407
Hanidziar D, Robson SC (2021) Synapomorphic features of hepatic and pulmonary vasculatures include comparable purinergic signaling responses in host defense and modulation of inflammation. Am J Physiol 321(2):G200–G212
Heidari R, Niknahad H (2019) The role and study of mitochondrial impairment and oxidative stress in cholestasis. In: Vinken M (ed) Experimental cholestasis research. Springer, New York, NY, pp 117–132
Heidari R, Babaei H, Eghbal MA (2013) Cytoprotective effects of organosulfur compounds against methimazole induced toxicity in isolated rat hepatocytes. Adv Pharm Bull 3(1):135–142
Heidari R, Babaei H, Roshangar L, Eghbal MA (2014) Effects of enzyme induction and/or glutathione depletion on methimazole-induced hepatotoxicity in mice and the protective role of N-acetylcysteine. Adv Pharm Bull 4(1):21–28
Heidari R, Jamshidzadeh A, Keshavarz N, Azarpira N (2015a) Mitigation of methimazole-induced hepatic injury by taurine in mice. Sci Pharm 83(1):143–158
Heidari R, Niknahad H, Jamshidzadeh A, Azarpira N, Bazyari M, Najibi A (2015b) Carbonyl traps as potential protective agents against methimazole-induced liver injury. J Biochem Mol Toxicol 29(4):173–181
Heidari R, Esmailie N, Azarpira N, Najibi A, Niknahad H (2016a) Effect of thiol-reducing agents and antioxidants on sulfasalazine-induced hepatic injury in normotermic recirculating isolated perfused rat liver. Toxicol Res 32(2):133–140
Heidari R, Jamshidzadeh A, Niknahad H, Safari F, Azizi H, Abdoli N, Ommati MM, Khodaei F, Saeedi A, Najibi A (2016c) The hepatoprotection provided by taurine and glycine against antineoplastic drugs induced liver injury in an ex vivo model of normothermic recirculating isolated perfused rat liver. Trend Pharm Sci 2(1):59–76
Heidari R, Rasti M, Shirazi Yeganeh B, Niknahad H, Saeedi A, Najibi A (2016d) Sulfasalazine-induced renal and hepatic injury in rats and the protective role of taurine. Bioimpacts 6(1):3–8
Heidari R, Moezi L, Asadi B, Ommati MM, Azarpira N (2017) Hepatoprotective effect of boldine in a bile duct ligated rat model of cholestasis/cirrhosis. PharmaNutrition 5(3):109–117
Heidari R, Jafari F, Khodaei F, Shirazi Yeganeh B, Niknahad H (2018b) Mechanism of valproic acid-induced Fanconi syndrome involves mitochondrial dysfunction and oxidative stress in rat kidney. Nephrology 23(4):351–361
Heidari R, Jafari F, Khodaei F, Yeganeh BS, Niknahad H (2018c) Mechanism of valproic acid-induced Fanconi syndrome involves mitochondrial dysfunction and oxidative stress in rat kidney. Nephrology 23(4):351–361
Heidari R, Jamshidzadeh A, Ghanbarinejad V, Ommati MM, Niknahad H (2018d) Taurine supplementation abates cirrhosis-associated locomotor dysfunction. Clin Exp HEPATOL 4(2):72–82
Heidari R, Arabnezhad MR, Ommati MM, Azarpira N, Ghodsimanesh E, Niknahad H (2019a) Boldine supplementation regulates mitochondrial function and oxidative stress in a rat model of hepatotoxicity. Pharm Sci 25(1):1–10
Heidari R, Jamshidzadeh A, Ommati MM, Rashidi E, Khodaei F, Sadeghi A, Hosseini A, Niknahad H (2019c) Ammonia-induced mitochondrial impairment is intensified by manganese co-exposure: relevance to the management of subclinical hepatic encephalopathy and cirrhosis-associated brain injury. Clin Exp HEPATOL 5(2):109–117
Heidari R, Mohammadi H, Ghanbarinejad V, Ahmadi A, Ommati MM, Niknahad H, Jamshidzadeh A, Azarpira N, Abdoli N (2019e) Proline supplementation mitigates the early stage of liver injury in bile duct ligated rats. J Basic Clin Physiol Pharmacol 30(1):91–101
Heidari R, Ahmadi A, Ommati MM, Niknahad H (2020) Methylene Blue improves mitochondrial function in the liver of cholestatic rats. Trend Pharm Sci 6(2):73–86
Heidari R, Jamshidzadeh A, Niknahad H, Mardani E, Ommati MM, Azarpira N, Khodaei F, Zarei A, Ayarzadeh M, Mousavi S, Abdoli N, Yeganeh BS, Saeedi A, Najibi A (2016b) Effect of taurine on chronic and acute liver injury: focus on blood and brain ammonia. Toxicol Rep 3:870–879
Heidari R, Ghanbarinejad V, Mohammadi H, Ahmadi A, Esfandiari A, Azarpira N, Niknahad H (2018a) Dithiothreitol supplementation mitigates hepatic and renal injury in bile duct ligated mice: potential application in the treatment of cholestasis-associated complications. Biomed Pharmacother 99:1022–1032
Heidari R, Behnamrad S, Khodami Z, Ommati MM, Azarpira N, Vazin A (2019b) The nephroprotective properties of taurine in colistin-treated mice is mediated through the regulation of mitochondrial function and mitigation of oxidative stress. Biomed Pharmacother 109:103–111
Heidari R, Mandegani L, Ghanbarinejad V, Siavashpour A, Ommati MM, Azarpira N, Najibi A, Niknahad H (2019d) Mitochondrial dysfunction as a mechanism involved in the pathogenesis of cirrhosis-associated cholemic nephropathy. Biomed Pharmacother 109:271–280
Herraez E, Lozano E, Poli E, Keitel V, De Luca D, Williamson C, Marin JJG, Macias RIR (2014) Role of macrophages in bile acid-induced inflammatory response of fetal lung during maternal cholestasis. J Mol Med 92(4):359–372
Hipkiss AR, Preston JE, Himsworth DTM, Worthington VC, Keown M, Michaelis J, Lawrence J, Mateen A, Allende L, Eagles PAM et al (1998) Pluripotent protective effects of carnosine, a naturally occurring dipeptidea. Ann N Y Acad Sci 854(1):37–53
Hipkiss AR, Brownson C, Carrier MJ (2001) Carnosine, the anti-ageing, anti-oxidant dipeptide, may react with protein carbonyl groups. Mech Ageing Dev 122(13):1431–1445
Hu Z-H, Kong Y-Y, Ren J-J, Huang T-J, Wang Y-Q, Liu L-X (2020) Kidney and lung tissue modifications after BDL-induced liver injury in mice are associated with increased expression of IGFBPrP1 and activation of the NF-κB inflammation pathway. Int J Clin Exp Pathol 13(2):192–202
Impellizzeri D, Siracusa R, Cordaro M, Peritore AF, Gugliandolo E, D’amico R, Fusco R, Crupi R, Rizzarelli E, Cuzzocrea S, Vaccaro S, Pulicetta M, Greco V, Sciuto S, Schiavinato A, Messina L, Di Paola R (2020) Protective effect of a new hyaluronic acid -carnosine conjugate on the modulation of the inflammatory response in mice subjected to collagen-induced arthritis. Biomed Pharmacother 125110023
Jaeschke H, Hasegawa T (2006) Role of neutrophils in acute inflammatory liver injury. Liver Int 26(8):912–919
Jamshidzadeh A, Abdoli N, Niknahad H, Azarpira N, Mardani E, Mousavi S, Abasvali M, Heidari R (2017a) Taurine alleviates brain tissue markers of oxidative stress in a rat model of hepatic encephalopathy. Trend Pharm Sci 3(3):181–192
Jamshidzadeh A, Heidari R, Latifpour Z, Ommati MM, Abdoli N, Mousavi S, Azarpira N, Zarei A, Zarei M, Asadi B, Abasvali M, Yeganeh Y, Jafari F, Saeedi A, Najibi A, Mardani E (2017c) Carnosine ameliorates liver fibrosis and hyperammonemia in cirrhotic rats. Clin Res Hepatol Gastroenterol 41(4):424–434
Jamshidzadeh A, Heidari R, Abasvali M, Zarei M, Ommati MM, Abdoli N, Khodaei F, Yeganeh Y, Jafari F, Zarei A, Latifpour Z, Mardani E, Azarpira N, Asadi B, Najibi A (2017b) Taurine treatment preserves brain and liver mitochondrial function in a rat model of fulminant hepatic failure and hyperammonemia. Biomed Pharmacother 86:514–520
Jukić I, Kolobarić N, Stupin A, Matić A, Kozina N, Mihaljević Z, Mihalj M, Šušnjara P, Stupin M, Ćurić ŽB, Selthofer-Relatić K, Kibel A, Lukinac A, Kolar L, Kralik G, Kralik Z, Széchenyi A, Jozanović M, Galović O, Medvidović-Kosanović M, Drenjančević I (2021) Carnosine, small but mighty—prospect of use as functional ingredient for functional food formulation. Antioxidants 10(7):1037
Jüngst C, Lammert F (2013) Cholestatic liver disease. Dig Dis 31(1):152–154
Klebanov GI, Teselkin YuO, n, Babenkova IV, Popov IN, Levin G, Tyulina OV, Boldyrev AA, Vladimirov YuA n, (1997) Evidence for a direct interaction of superoxide anion radical with carnosine. Biochem Mol Biol Int 43(1):99–106
Kohen R, Yamamoto Y, Cundy KC, Ames BN (1988) Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci U S A 85(9):3175–3179
Kubota M, Kobayashi N, Sugizaki T, Shimoda M, Kawahara M, Tanaka K-i (2020) Carnosine suppresses neuronal cell death and inflammation induced by 6-hydroxydopamine in an in vitro model of Parkinson’s disease. PLoS ONE 15(10):e0240448
Kuloglu N, Sönmez MF (2015) A biochemical and immunohistochemical study of the protective effects of carnosine for carbon tetrachloride induced liver injury in rats. Biotech Histochem 90(8):608–614
Lee JW, Miyawaki H, Bobst EV, Hester JD, Ashraf M, Bobst AM (1999) Improved functional recovery of ischemic rat hearts due to singlet oxygen scavengers histidine and carnosine. J Mol Cell Cardiol 31(1):113–121
Lee Y-t, Hsu C-c, Lin M-h, Liu K-s, Yin M-c (2005) Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur J Pharmacol 513(1):145–150
Li T, Chiang JYL (2017) Bile acid-induced liver injury in cholestasis. In: Ding W-X, Yin X-M (eds) Cellular injury in liver diseases. Springer International Publishing, Cham, pp 143-172
Motoyama T, Okamoto K, Kukita I, Hamaguchi M, Kinoshita Y, Ogawa H (2003) Possible role of increased oxidant stress in multiple organ failure after systemic inflammatory response syndrome. Crit Care Med 31(4):1048–1052
Mousavi K, Niknahad H, Ghalamfarsa A, Mohammadi H, Azarpira N, Ommati MM, Heidari R (2020) Taurine mitigates cirrhosis-associated heart injury through mitochondrial-dependent and antioxidative mechanisms. Clin Exp HEPATOL 6(3):207–219
Mousavi K, Niknahad H, Li H, Jia Z, Manthari RK, Zhao Y, Shi X, Chen Y, Ahmadi A, Azarpira N, Khalvati B, Ommati MM, Heidari R (2021) The activation of nuclear factor-E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling blunts cholestasis-induced liver and kidney injury. Toxicol Res 10(4):911–927
Najibi A, Rezaei H, Manthari RK, Niknahad H, Jamshidzadeh A, Farshad O, Yan F, Ma Y, Xu D, Tang Z, Ommati MM, Heidari R (2022) Cellular and mitochondrial taurine depletion in bile duct ligated rats: a justification for taurine supplementation in cholestasis/cirrhosis. Clin Exp HEPATOL 8(3):195–210
Niknahad H, Heidari R, Alzuhairi AM, Najibi A (2015) Mitochondrial dysfunction as a mechanism for pioglitazone-induced injury toward HepG2 cell line. Pharm Sci 20(4):169–174
Niknahad H, Heidari R, Mohammadzadeh R, Ommati MM, Khodaei F, Azarpira N, Abdoli N, Zarei M, Asadi B, Rasti M, Yeganeh BS, Taheri V, Saeedi A, Najibi A (2017) Sulfasalazine induces mitochondrial dysfunction and renal injury. Ren Fail 39(1):745–753
Okada S, Hasegawa S, Hasegawa H, Ainai A, Atsuta R, Ikemoto K, Sasaki K, Toda S, Shirabe K, Takahara M, Harada S, Morishima T, Ichiyama T (2013) Analysis of bronchoalveolar lavage fluid in a mouse model of bronchial asthma and H1N1 2009 infection. Cytokine 63(2):194–200
Ommati MM, Jamshidzadeh A, Niknahad H, Mohammadi H, Sabouri S, Heidari R, Abdoli N (2017) N-acetylcysteine treatment blunts liver failure-associated impairment of locomotor activity. PharmaNutrition 5(4):141–147
Ommati MM, Heidari R, Ghanbarinejad V, Abdoli N, Niknahad H (2019c) Taurine treatment provides neuroprotection in a mouse model of manganism. Biol Trace Elem Res 190(2):384–395
Ommati MM, Heidari R, Ghanbarinejad V, Aminian A, Abdoli N, Niknahad H (2020b) The neuroprotective properties of carnosine in a mouse model of manganism is mediated via mitochondria regulating and antioxidative mechanisms. Nutr Neurosci 23(9):731–743
Ommati MM, Heidari R, Zamiri MJ, Sabouri S, Zaker L, Farshad O, Jamshidzadeh A, Mousapour S (2020c) The footprints of oxidative stress and mitochondrial impairment in arsenic trioxide-induced testosterone release suppression in pubertal and mature F1-male BALB/c mice via the downregulation of 3β-HSD, 17β-HSD, and CYP11a expression. Biol Trace Elem Res 195(1):125–134
Ommati MM, Amjadinia A, Mousavi K, Azarpira N, Jamshidzadeh A, Heidari R (2021a) N-acetyl cysteine treatment mitigates biomarkers of oxidative stress in different tissues of bile duct ligated rats. Stress 24(2):213–228
Ommati MM, Attari H, Siavashpour A, Shafaghat M, Azarpira N, Ghaffari H, Moezi L, Heidari R (2021b) Mitigation of cholestasis-associated hepatic and renal injury by edaravone treatment: evaluation of its effects on oxidative stress and mitochondrial function. Liver Research 5(3):181–193
Ommati MM, Mohammadi H, Mousavi K, Azarpira N, Farshad O, Dehghani R, Najibi A, Kamran S, Niknahad H, Heidari R (2021c) Metformin alleviates cholestasis-associated nephropathy through regulating oxidative stress and mitochondrial function. Liver Res 5(3):171–180
Ommati MM, Jamshidzadeh A, Saeed M, Rezaei M, Heidari R (2022b) Dextromethorphan improves locomotor activity and decreases brain oxidative stress and inflammation in an animal model of acute liver failure. Clin Exp HEPATOL 8(3):178–187
Ommati MM, Li H, Jamshidzadeh A, Khoshghadam F, Retana-Márquez S, Lu Y, Farshad O, Nategh Ahmadi MH, Gholami A, Heidari R (2022c) The crucial role of oxidative stress in non-alcoholic fatty liver disease-induced male reproductive toxicity: the ameliorative effects of Iranian indigenous probiotics. Naunyn-Schmiedeberg’s Arch Pharmacol 395(2):247–265
Ommati MM, Mobasheri A, Ma Y, Xu D, Tang Z, Manthari RK, Abdoli N, Azarpira N, Lu Y, Sadeghian I, Mousavifaraz A, Nadgaran A, Nikoozadeh A, Mazloomi S, Mehrabani PS, Rezaei M, Xin H, Mingyu Y, Niknahad H, Heidari R (2022d) Taurine mitigates the development of pulmonary inflammation, oxidative stress, and histopathological alterations in a rat model of bile duct ligation. Naunyn-Schmiedeberg’s Arch Pharmacol 395(12):1557–1572
Ommati MM, Farshad O, Jamshidzadeh A, Heidari R (2019a) Taurine enhances skeletal muscle mitochondrial function in a rat model of resistance training. PharmaNutrition 9100161
Ommati MM, Farshad O, Niknahad H, Arabnezhad MR, Azarpira N, Mohammadi HR, Haghnegahdar M, Mousavi K, Akrami S, Jamshidzadeh A, Heidari R (2019b) Cholestasis-associated reproductive toxicity in male and female rats: the fundamental role of mitochondrial impairment and oxidative stress. Toxicol Lett 316:60–72
Ommati MM, Heidari R, Manthari RK, Tikka Chiranjeevi S, Niu R, Sun Z, Sabouri S, Zamiri MJ, Zaker L, Yuan J, Wang J, Zhang J, Wang J (2019d) Paternal exposure to arsenic resulted in oxidative stress, autophagy, and mitochondrial impairments in the HPG axis of pubertal male offspring. Chemosphere 236:124325
Ommati MM, Farshad O, Mousavi K, Jamshidzadeh A, Azmoon M, Heidari S, Azarpira N, Niknahad H, Heidari R (2020a) Betaine supplementation mitigates intestinal damage and decreases serum bacterial endotoxin in cirrhotic rats. PharmaNutrition 12:100179
Ommati MM, Shi X, Li H, Zamiri MJ, Farshad O, Jamshidzadeh A, Heidari R, Ghaffari H, Zaker L, Sabouri S, Chen Y (2020d) The mechanisms of arsenic-induced ovotoxicity, ultrastructural alterations, and autophagic related paths: an enduring developmental study in folliculogenesis of mice. Ecotoxicol Environ Saf 204:110973
Ommati MM, Abdoli N, Firoozi M, Akhlagh A, Mazloomi S, Mousavi K, Niknahad H, Heidari R (2022a) Sildenafil blunts lung inflammation and oxidative stress in a rat model of cholestasis. Pharm Sci In-Press
Patil A, Mayo MJ (2008) Complications of cholestasis. In: Md KDL, Md JAT (eds) Cholestatic liver disease. Humana Press, pp 155-169
Prokopieva VD, Yarygina EG, Bokhan NA, Ivanova SA (2016) Use of carnosine for oxidative stress reduction in different pathologies. Oxid Med Cell Longev 2016:2939087
Raffo AJ, Perlman H, Chen M-W, Day ML, Streitman JS, Buttyan R (1995) Overexpression of Bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res 55(19):4438–4445
Richter K, Konzack A, Pihlajaniemi T, Heljasvaara R, Kietzmann T (2015) Redox-fibrosis: impact of TGFβ1 on ROS generators, mediators and functional consequences. Redox Biol 6:344–352
Rodríguez-Garay EA (2003) Cholestasis: human disease and experimental animal models. Ann Hepatol 2(4):150–158
Shafaroodi H, Ebrahimi F, Moezi L, Hashemi M, Doostar Y, Ghasemi M, Dehpour AR (2010) Cholestasis induces apoptosis in mice cardiac cells: the possible role of nitric oxide and oxidative stress. Liver Int 30(6):898–905
Shafiekhani M, Ommati MM, Azarpira N, Heidari R, Salarian AA (2019) Glycine supplementation mitigates lead-induced renal injury in mice. J Exp Pharmacol 11:15–22
Shikata F, Sakaue T, Nakashiro K-i, Okazaki M, Kurata M, Okamura T, Okura M, Ryugo M, Nakamura Y, Yasugi T, Higashiyama S, Izutani H (2014) Pathophysiology of lung injury induced by common bile duct ligation in mice. PLoS ONE 9(4):e94550
Siavashpour A, Khalvati B, Azarpira N, Mohammadi H, Niknahad H, Heidari R (2020) Poly (ADP-ribose) polymerase-1 (PARP-1) overactivity plays a pathogenic role in bile acids-induced nephrotoxicity in cholestatic rats. Toxicol Lett 330:144–158
Soliman KM, Abdul-Hamid M, Othman AI (2007) Effect of carnosine on gentamicin-induced nephrotoxicity. Med Sci Technol 13(3):BR73-BR83
Sun C, Wu Q, Zhang X, He Q, Zhao H (2017) Mechanistic evaluation of the protective effect of carnosine on acute lung injury in sepsis rats. Pharmacology 100(5–6):292–300
Tanaka K-I, Sugizaki T, Kanda Y, Tamura F, Niino T, Kawahara M (2017b) Preventive effects of carnosine on lipopolysaccharide-induced lung injury. Sci Rep 7(1):42813
Tanaka K-I, Sugizaki T, Kanda Y, Tamura F, Niino T, Kawahara M (2017a) Preventive effects of carnosine on lipopolysaccharide-induced lung injury. Sci Rep 7:42813
Thimmulappa RK, Chattopadhyay I, Rajasekaran S (2019) Oxidative stress mechanisms in the pathogenesis of environmental lung diseases. Oxid Stress Lung Dis 103–137
Tsai S-J, Kuo W-W, Liu W-H, Yin M-C (2010) Antioxidative and anti-inflammatory protection from carnosine in the striatum of MPTP-treated mice. J Agric Food Chem 58(21):11510–11516
Vistoli G, Colzani M, Mazzolari A, Gilardoni E, Rivaletto C, Carini M, Aldini G (2017) Quenching activity of carnosine derivatives towards reactive carbonyl species: focus on α-(methylglyoxal) and β-(malondialdehyde) dicarbonyls. Biochem Biophys Res Commun 492(3):487–492
Wynn TA, Ramalingam TR (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18(7):1028–1040
Xie J, Yu J, Zhang Z, Liu D, Fan Y, Wu Y, Ma H, Wang C, Hong Z (2022) AMPK pathway is implicated in low level lead-induced pubertal testicular damage via disordered glycolysis. Chemosphere 291:132819
Xu T, Wang C, Zhang R, Xu M, Liu B, Wei D, Wang G, Tian S (2015) Carnosine markedly ameliorates H9N2 swine influenza virus-induced acute lung injury. J Gen Virol 96(Pt 10):2939–2950
Yalaza M, Akin I, Altiner S, Ayral PA, Yazihan N (2022) Is carnosine effective to alleviate lung injury: a systematic review. Turk J Biochem 47(1):1–7
Yan S-l, Wu S-t, Yin M-c, Chen H-t, Chen H-c (2009) Protective effects from carnosine and histidine on acetaminophen-induced liver injury. J Food Sci 74(8):259–265
Yu L, Ding Y, Huang T, Huang X (2014) Effect of bile acid on fetal lung in rat model of intrahepatic cholestasis of pregnancy. Int J Endocrinol 2014:e308274
Zecca E, Costa S, Lauriola V, Vento G, Papacci P, Romagnoli C (2004) Bile acid pneumonia: a “new” form of neonatal respiratory distress syndrome? Pediatrics 114(1):269–272
Zecca E, De Luca D, Baroni S, Vento G, Tiberi E, Romagnoli C (2008) Bile acid-induced lung injury in newborn infants: a bronchoalveolar lavage fluid study. Pediatrics 121(1):e146-149
Funding
Natural Science Foundation of Shanxi financially supported this project (20210302124411). Grants 23040/23028/23031/23701 from the Vice-Chancellor of Research Affairs, Shiraz University of Medical Sciences, Shiraz, Iran, were also used for this investigation.
Author information
Authors and Affiliations
Contributions
M.M. Ommati, R. Heidari, N. Azarpira, A. Ghiasvand, and H. Niknahad were involved in funding acquisition, subject conceptualization, validation, methodology, data analysis, resources management, project administration, and supervision, writing the original draft, and review and editing the manuscript. S. Sabouri, A. Arjmand, S. Alidaee, S. Mazloomi, A. Najibi, A. Nikoozadeh, P. Ahmadi, H. Rezaei, F. Khodaei, and N. Abdoli were involved in the literature review, data visualization, data analysis, and writing the manuscript draft. S. Alidaee, S. Mazloomi, A. Najibi, A. Nikoozadeh, P. Ahmadi, H. Rezaei, and F. Khodaei were involved in data collection. All authors read and confirmed the final version of this manuscript. The authors announced that all data were generated in-house, and no paper mill was used.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The institutional ethics committee approved all experimental animal procedures at Shiraz University of Medical Sciences, Shiraz, Iran (IR.SUMS.REC.1399.1353). This study does not include any human participants.
Consent to participate
Not applicable. This study contains no human data.
Consent for publication
Not applicable. This study contains no human data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ommati, M.M., Sabouri, S., Niknahad, H. et al. Pulmonary inflammation, oxidative stress, and fibrosis in a mouse model of cholestasis: the potential protective properties of the dipeptide carnosine. Naunyn-Schmiedeberg's Arch Pharmacol 396, 1129–1142 (2023). https://doi.org/10.1007/s00210-023-02391-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02391-y