Abstract
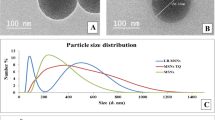
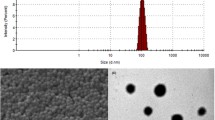
Magnesium (Mg2+) is the fourth most abundant cation in the human body and is involved in maintaining varieties of cellular and neurological functions. Magnesium deficiency has been associated with numerous diseases, particularly neurological disorders, and its supplementation has proven beneficial. However, magnesium therapy in neurological diseases is limited because of the inability of magnesium to cross the blood–brain barrier (BBB). The present study focuses on developing magnesium sulphate nanoparticles (MGSN) to improve blood–brain barrier permeability. MGSN was prepared by precipitation technique with probe sonication. The developed formulation was characterized by DLS, EDAX, FT-IR and quantitative and qualitative estimation of magnesium. According to the DLS report, the average size of the prepared MGSN is found to be 247 nm. The haemocompatibility assay studies revealed that the prepared MGSN are biocompatible at different concentrations. The in vitro BBB permeability assay conducted by Parallel Artificial Membrane Permeability Assay (PAMPA) using rat brain tissue revealed that the prepared MGSN exhibited enhanced BBB permeability as compared to the marketed i.v. MgSO4 injection. The reversal effect of MGSN to digoxin-induced Na+/K+ ATPase enzyme inhibition using brain microslices confirmed that MGSN could attenuate the altered levels of Na+ and K+ and is useful in treating neurological diseases with altered expression of Na+/K+ ATPase activity.


Similar content being viewed by others
Data Availability
It is confirmed that the data supporting the findings of the study are available within the article.
References
Ahmed, Faheemuddin, Abdul Mohammed (2019) Magnesium: The forgotten electrolyte-a review on hypomagnesemia. Med Sci (Basel, Switzerland) 7 (4). https://doi.org/10.3390/medsci7040056
Alawi, Abdullah M Al, Sandawana William Majoni, Henrik Falhammar (2018) Magnesium and human health: perspectives and research directions. Int J Endocrinol 2018. https://doi.org/10.1155/2018/9041694
Arnaiz Georgina Rodríguez, de Lores, and María Graciela López Ordieres (2014) Brain Na+, K+-ATPase activity in aging and disease. Int J Biomed Sci 10(2):85–102 (/pmc/articles/PMC4092085/)
Bhaskar RK, Vaijayanthi V, Charulatha RS Brito R, Mohanambal E, Madhusudan Rao Y (2011) Nanoparticles for brain targeting. Ind J Novel Drug Deliv 3(2):91–97. https://www.researchgate.net/publication/257820237_Nanoparticles_for_Brain_Targeting
Bicker J, Alves G, Fortuna A, Soares-da-Silva P, Falcão A (2016) A new PAMPA model using an in-house brain lipid extract for screening the blood-brain barrier permeability of drug candidateS. Int J Pharm 501(1–2):102–111. https://doi.org/10.1016/j.ijpharm.2016.01.074
Botturi A, Ciappolino V, Delvecchio G, Boscutti A, Viscardi B, Brambilla P (2020) The role and the effect of magnesium in mental disorders: a systematic review. Nutrients 12(6):1–21. https://doi.org/10.3390/NU12061661
Brewer RP, Parra A, Borel CO, Hopkins MB, Reynolds JD (2001) Intravenous magnesium sulfate does not increase ventricular CSF ionized magnesium concentration of patients with intracranial hypertension. Clin Neuropharmacol 24(6):341–345. https://doi.org/10.1097/00002826-200111000-00005
Busingye DS, Turner RJ, Vink R (2016) Combined magnesium/polyethylene glycol facilitates the neuroprotective effects of magnesium in traumatic brain injury at a reduced magnesium dose. CNS Neurosci Ther 22(10):854–859. https://doi.org/10.1111/cns.12591
Chen S, Yan Xu, He X, Ying Su, Yang J, Chen W, Tan H (2019) Microemulsion synthesis of nanosized calcium sulfate hemihydrate and its morphology control by different surfactants. ACS Omega 4(5):9552–9556. https://doi.org/10.1021/ACSOMEGA.9B00797/SUPPL_FILE/AO9B00797_SI_001.PDF
Chi, Cheng Ting, Ming Han Lee, Ching Feng Weng, Max K Leong (2019) In silico prediction of PAMPA effective permeability using a two-QSAR approach. Int J Molecular Sci 20 (13). https://doi.org/10.3390/ijms20133170
Daneman, R, Prat A (2015) The blood–brain barrier. Cold Spring Harbor Perspect Biol 7 (1). https://doi.org/10.1101/cshperspect.a020412
Divya G, Panonnummal R, Swati Gupta R, Jayakumar, and M. Sabitha. (2016) Acitretin and aloe-emodin loaded chitin nanogel for the treatment of psoriasis. Eur J Pharm Biopharm 107:97–109. https://doi.org/10.1016/j.ejpb.2016.06.019
Fischer PWF, Giroux A (1987) Effects of dietary magnesium on sodium-potassium pump action in the heart of rats. J Nutr 117(12):2091–2095. https://doi.org/10.1093/JN/117.12.2091
Fuchs-Buder T, Tramèr MR, Tassonyi E (1997) Cerebrospinal fluid passage of intravenous magnesium sulfate in neurosurgical patients. J Neurosurg Anesthesiol. https://doi.org/10.1097/00008506-199710000-00006
Ghorbani HR, Mehr FP, Pazoki H, Rahmani BM (2015) Synthesis of ZnO nanoparticles by precipitation method. Orient J Chem https://doi.org/10.13005/ojc/310281
Gupta, VK, Saver JL, Kidwell C, Eckstein M, Starkman S (2004) Intravenous magnesium for neuroprotection in acute stroke: clinical hope versus basic neuropharmacology [6] (Multiple Letters). Stroke. Lippincott Williams and Wilkins. https://doi.org/10.1161/01.STR.0000147971.14988.50
Hallak M, Berman RF, Irtenkauf SM, Evans MI, Cotton DB (1992) Peripheral magnesium sulfate enters the brain and increases the threshold for hippocampal seizures in rats. Am J Obstet Gynecol 167(6):1605–1610. https://doi.org/10.1016/0002-9378(92)91749-Z
Jain T, Kumar S, Dutta PK (2016) Dibutyrylchitin nanoparticles as novel drug carrieR. Int J Biol Macromol 82(January):1011–1017. https://doi.org/10.1016/j.ijbiomac.2015.11.031
Jahnen-Dechent, Wilhelm, Markus Ketteler (2012) Magnesium basics. CKJ: Clin Kidney J 5 (SUPPL. 1). https://doi.org/10.1093/ndtplus/sfr163
Jeon JS, Sheen SH, Hwang G, Kang SH, Heo DH, Cho YJ (2012) Intravenous magnesium infusion for the prevention of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Journal of Korean Neurosurgical Society 52(2):75–79. https://doi.org/10.3340/jkns.2012.52.2.75
Jin X, Liu MY, Zhang DF, Gao H, Wei MJ (2018) Elevated circulating magnesium levels in patients with Parkinson’s disease: a meta-analysis. Neuropsychiatr Dis Treat 14:3159–3168. https://doi.org/10.2147/NDT.S186209
Kesmati Mahnaz, Konani Maryam, Torabi Mozhgan, Khajehpour Lotfollah (2016) Magnesium oxide nanoparticles reduce anxiety induced by morphine withdrawal in adult male mice. Physiol Pharmacol 20(2016):197–205
Kim YS, Won YJ, Lim BG, Min TJ, Kim YH, Lee IO (2020) Neuroprotective effects of magnesium L-threonate in a hypoxic zebrafish model. BMC Neurosci 21(1):1–11. https://doi.org/10.1186/s12868-020-00580-6
Kirkland, AE, Sarlo GL, Holton KF (2018) The role of magnesium in neurological disorders. Nutrients 10 (6). https://doi.org/10.3390/NU10060730
Kumar VS, Vinayan KP, Sabitha M (2022) Anticonvulsant and acute toxicity evaluation of phenytoin sodium–loaded polymeric nanomicelle in MES rat model. J Nanopart Res 24(8):154. https://doi.org/10.1007/S11051-022-05502-7
Laur N, Kinscherf R, Pomytkin K, Kaiser L, Knes O, Deigner HP (2020) ICP-MS trace element analysis in serum and whole blood. PLoS ONE 15(5):e0233357. https://doi.org/10.1371/journal.pone.0233357
Lee JHT, Roy J, Sohn HM, Cheong Mi, Liu J, Stammers AT, Tetzlaff W, Kwon BK (2010) Magnesium in a polyethylene glycol formulation provides neuroprotection after unilateral cervical spinal cord injury. Spine 35(23):2041–2048. https://doi.org/10.1097/BRS.0b013e3181d2d6c5
Li W, Jia Yu, Liu Y, Huang X, Abumaria N, Zhu Y, Huang X et al (2013) Elevation of brain magnesium prevents and reverses cognitive deficits and synaptic loss in Alzheimer’s disease mouse model. J Neurosci 33(19):8423–8441. https://doi.org/10.1523/JNEUROSCI.4610-12.2013
Liu D, Lin B, Shao W, Zhu Z, Ji T, Yang C (2014) In vitro and in vivo studies on the transport of PEGylated silica nanoparticles across the blood-brain barrier. ACS Appl Mater Interfaces 6(3):2131–2136. https://doi.org/10.1021/am405219u
Lombardo SM, Schneider M, Türeli AE, Türeli NG (2020) Key for crossing the BBB with nanoparticles: the rational design. Beilstein J Nanotechnol 11:866. https://doi.org/10.3762/BJNANO.11.72
Maier JA, Pickering G, Giacomoni E, Cazzaniga A, Pellegrino P (2020) Headaches and magnesium: mechanisms, bioavailability, therapeutic efficacy and potential advantage of magnesium pidolate. Nutrients 12(9):2660. https://doi.org/10.3390/nu12092660
Mathew AA, Panonnummal R (2021) ‘Magnesium’-the master cation-as a drug—possibilities and evidences. Biometals. https://doi.org/10.1007/s10534-021-00328-7
McKee J, Andrew RP, Brewer GE, Macy B-B, Campbell KA, Borel CO, Reynolds JD, Warner DS (2005) Analysis of the brain bioavailability of peripherally administered magnesium sulfate: a study in humans with acute brain injury undergoing prolonged induced hypermagnesemia. Crit Care Med 33(3):661–666. https://doi.org/10.1097/01.CCM.0000156293.35868.B2
Mohan Dixit C, Suresh Akhil, Mukundan Shilpa, Gupta Swati, Viswanad Vidya (2018) Development and in vitro evaluation of nanolipid carriers of clobetasol propionate and pramoxine hydrochloride for topical delivery. Int J Appl Pharm 10(3):28–36. https://doi.org/10.22159/ijap.2018v10i3.24171
Mounir GN, Vink R (2011) Magnesium transport across the blood-brain barriers. In Magnesium in the Central Nervous System, 59–74. University of Adelaide Press. https://doi.org/10.1017/UPO9780987073051.004
Nellore J, Pauline C, Amarnath K (2013) Bacopa monnieri phytochemicals mediated synthesis of platinum nanoparticles and its neurorescue effect on 1- methyl 4-phenyl 1,2,3,6 tetrahydropyridine-induced experimental parkinsonism in zebrafish. Journal of Neurodegenerative Diseases 2013(March):1–8. https://doi.org/10.1155/2013/972391
Ovalles F, Gallignani M, Rondón R, Brunetto MR, Luna R (2009) Determination of sulphate for measuring magnesium sulphate in pharmaceuticals by flow analysis-fourier transforms infrared spectroscopy. Lat Am J Pharm 28(2):173–182
ParkJames SA, LearyJaehong Key F (2019) Top-down fabrication-based nano/microparticles for molecular imaging and drug delivery. Dove Press 14:6631–6644. https://doi.org/10.2147/IJN.S212037
Rajendran, Rajalakshmi, Krishnakumar Neelakandha Menon, Sreeja Chandrasekharan Nair (2021) Nanotechnology approaches for enhanced CNS drug delivery in the management of schizophrenia. Adv Pharm Bull. https://doi.org/10.34172/apb.2022.052
Ratge Dieter, Kohse Klaus P, Wisser Hermann (1986) Measurement of magnesium in serum and urine with a random access analyzer by use of a modified xylidyl blue-1 procedure. Clin Chim Acta; Int J Clin Chem 159(2):197–203. https://doi.org/10.1016/0009-8981(86)90052-5
Romeo V, Cazzaniga A, Maier JAM (2019) Magnesium and the blood-brain barrier in vitro: effects on permeability and magnesium transport. Magnes Res 32(1):16–24. https://doi.org/10.1684/mrh.2019.0454
Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L (2016) Nanoparticle-mediated brain drug delivery: overcoming blood-brain barrier to treat neurodegenerative diseases. J Control Release 235(August):34–47. https://doi.org/10.1016/j.jconrel.2016.05.044
Saver JL, Starkman S, Eckstein M, Stratton SJ, Pratt FD, Hamilton S, Conwit R et al (2015) Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med 372(6):528–536. https://doi.org/10.1056/NEJMoa1408827
Seko Indrit, Şahin Adem, Tonbul Hayrettin, Çapan Yılmaz (2020) Brain-targeted nanoparticles to overcome the blood-brain barrier. J Pharm Technol 1(1):25–39. https://doi.org/10.37662/JPT.2020.4
Sen AP, Gulati A (2010) Use of magnesium in traumatic brain injury. Neurotherapeutics 7(1):91–99. https://doi.org/10.1016/j.nurt.2009.10.014
Shen Y, Dai L, Tian H, Runnan Xu, Li F, Li Z, Zhou J, Wang L, Dong J, Sun L (2019) Treatment of magnesium-l-threonate elevates the magnesium level in the cerebrospinal fluid and attenuates motor deficits and dopamine neuron loss in a mouse model of Parkinson’s disease. Neuropsychiatr Dis Treat 15:3143–3153. https://doi.org/10.2147/NDT.S230688
Skelding KA, Arellano JM, Powis DA, Rostas JA (2014) Excitotoxic stimulation of brain microslices as an in vitro model of stroke. Journal of Visualized Experiments : Jove, 84(February):e51291. https://doi.org/10.3791/51291
Sintov AC, Velasco-Aguirre C, Gallardo-Toledo E, Araya E, Kogan MJ (2016) Metal nanoparticles as targeted carriers circumventing the blood-brain barrier. Int Rev Neurobiol 130:199–227. https://doi.org/10.1016/BS.IRN.2016.06.007
Sifontes, ÁB, Cañizales E, Toro-Mendoza J, Ávila E, Hernández P, Delgado BA, Gutiérrez GB, Díaz Y, Cruz-Barrios E (2015) Obtaining highly crystalline barium sulphate nanoparticles via chemical precipitation and quenching in absence of polymer stabilizers. J Nanomater 2015. https://doi.org/10.1155/2015/510376
Urbańska, Kaja, Beata Pająk, Arkadiusz Orzechowski, Justyna Sokołowska, Marta Grodzik, Ewa Sawosz, Maciej Szmidt, Paweł Sysa (2015) The effect of silver nanoparticles (AgNPs) on proliferation and apoptosis of in ovo cultured glioblastoma multiforme (GBM) cells. Nanoscale Res Lett 10 (1). https://doi.org/10.1186/S11671-015-0823-5
Vink R (2016) Magnesium in the CNS: recent advances and developments. Magnes Res 29(3):95–101. https://doi.org/10.1684/mrh.2016.0408
Zehraa N Abdul-Ameer (2016) Effect of concentration on characterization of mgo nanoparticles using chemical bath method .Adv Nat Appl Sci 10(12) https://go.gale.com/ps/i.do?id=GALE%7CA488191825&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=19950772&p=AONE&sw=w&userGroupName=anon~2d2baf04
Acknowledgements
The authors are grateful to the ‘Amrita School of Pharmacy, Kochi, Kerala’ for providing all the infrastructure facilities to conduct the work as well as Dr. S K Kanthlal for the support and guidance in conducting the in vitro studies.
Funding
A seed grant fund supported this work from Amrita Vishwa Vidyapeetham with Seed Grant Number: K-PHAR-20–629.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study’s conception and design. SM and AAM performed the material preparation, data collection and analysis. The first draft of the manuscript was written by SM and AAM, and all the authors commented on previous versions. Supervision of the work and critical review of the article were done by RP. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experiments were carried out with the consent of the Amrita Vishwa Vidyapeetham Animal Care and Ethics Committee and in compliance with CPCSEA rules and regulations and the IAEC Code of Practice for the Care and Use of Animals for Scientific Purposes (Ref.no: IAEC/2019/3/6 Amend).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mathew, A.A., Mohapatra, S. & Panonnummal, R. Formulation and evaluation of magnesium sulphate nanoparticles for improved CNS penetrability. Naunyn-Schmiedeberg's Arch Pharmacol 396, 567–576 (2023). https://doi.org/10.1007/s00210-022-02356-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02356-7