Abstract
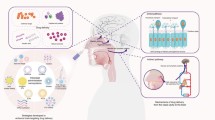
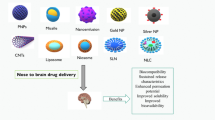
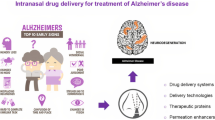
Neuroinflammation (NIF) plays an essential role in the pathology of neurological disorders like Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, and epilepsy. Despite progress in the drug discovery and development of new drugs, drug delivery to the central nervous system (CNS) still represents the challenge due to the presence of the blood–brain barrier (BBB). Targeting NIF may require an adequate amount of drug to cross the BBB. Recently, the intranasal (IN) drug administration has attracted increasing attention as a reliable method to cross the BBB and treat neurological disorders. On the other hand, using optimized nanoparticles may improve the IN delivery limitations, increase the mucoadhesive properties, and prevent drug degradation. NPs can carry and deliver drugs to the CNS by bypassing the BBB. In this review, we described briefly the NIF as a pathologic feature of CNS diseases. The potential treatment possibilities with IN transfer of NP-loaded drugs will enhance the establishment of more efficient nanoformulations and delivery systems.

Similar content being viewed by others
Data availability
No datasets were generated or analyzed during the current study.
References
Abbott NJ (2013) Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36:437–449
Adamczyk-Grochala J, Lewinska A (2020) Nano-based theranostic tools for the detection and elimination of senescent cells. Cells 9. https://doi.org/10.3390/cells9122659
Adams D, Joyce G, Richardson V, Ryman BE, Wiśniewski H (1977) Liposome toxicity in the mouse central nervous system. J Neurol Sci 31:173–179
Ahmad E et al (2017) Evidence of nose-to-brain delivery of nanoemulsions: cargoes but not vehicles. Nanoscale 9:1174–1183. https://doi.org/10.1039/c6nr07581a
Akbarzadeh A et al (2013) Liposome: classification, preparation, and applications. Nanoscale Res Lett 8:1–9
Alexander A et al (2019) Recent expansions of novel strategies towards the drug targeting into the brain. Int J Nanomed 14:5895
Amor S, Puentes F, Baker D, Van Der Valk P (2010) Inflammation in Neurodegenerative Diseases. Immunology 129:154–169
Aparicio-Blanco J, Romero IA, Male DK, Slowing K, García-García L, Torres-Suárez AI (2019) Cannabidiol enhances the passage of lipid nanocapsules across the blood–brain barrier both in vitro and in vivo. Mol Pharm 16:1999–2010
Barar J, Rafi MA, Pourseif MM, Omidi Y (2016) Blood-brain barrier transport machineries and targeted therapy of brain diseases. Bioimpacts 6:225–248. https://doi.org/10.15171/bi.2016.30
Battaglia L et al (2018) Lipid nanoparticles for intranasal administration: application to nose-to-brain delivery. Expert Opin Drug Deliv 15:369–378
Bhushan B, Bhushan B, Baumann (2007) Springer handbook of nanotechnology vol 2. Springer
Bony BA, Kievit FM (2019) A role for nanoparticles in treating traumatic brain injury. Pharmaceutics 11:473
Bourganis V, Kammona O, Alexopoulos A, Kiparissides C (2018a) Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur J Pharm Biopharm 128:337–362. https://doi.org/10.1016/j.ejpb.2018.05.009
Bourganis V, Kammona O, Alexopoulos A, Kiparissides CJEJOP, biopharmaceutics (2018) Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur J Pharm Biopharm 128:337–362
Bozzuto G, Molinari A (2015) Liposomes as nanomedical devices. Int J Nanomed 10:975
Bustamante-Marin XM, Ostrowski LE (2017) Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol 9:a028241. https://doi.org/10.1101/cshperspect.a028241
Cahalane C, Bonezzi J, Shelestak J, Clements R, Boika A, Yun YH, Shriver LP (2020) Targeted delivery of anti-inflammatory and imaging agents to microglial cells with polymeric nanoparticles. Mol Pharm 17:1816–1826
Caramella C, Ferrari F, Bonferoni M, Rossi S, Sandri G (2010) Chitosan and its derivatives as drug penetration enhancers. J Drug Deliv Sci Technol 20:5–13
Casals E, Gusta MF, Piella J, Casals G, Jiménez W, Puntes V (2017) Intrinsic and extrinsic properties affecting innate immune responses to nanoparticles: the case of cerium oxide. Front Immunol 8:970. https://doi.org/10.3389/fimmu.2017.00970
Charabati M, Rabanel JM, Ramassamy C, Prat A (2020) Overcoming the brain barriers: from immune cells to nanoparticles. Trends Pharmacol Sci 41:42–54. https://doi.org/10.1016/j.tips.2019.11.001
Chaturvedi M, Kumar M, Pathak K (2011) A review on mucoadhesive polymer used in nasal drug delivery system. J Adv Pharm Technol Res 2:215–222. https://doi.org/10.4103/2231-4040.90876
Chen H, Yang GZX, Getachew H, Acosta C, Sánchez CS, Konofagou EE (2016) Focused ultrasound-enhanced intranasal brain delivery of brain-derived neurotrophic factor. Sci Rep 6:1–8
Chowdhury HH, Cerqueira SR, Sousa N, Oliveira JM, Reis RL, Zorec R (2018) The uptake, retention and clearance of drug-loaded dendrimer nanoparticles in astrocytes - electrophysiological quantification. Biomater Sci 6:388–397. https://doi.org/10.1039/c7bm00886d
Chung EP, Cotter JD, Prakapenka AV, Cook RL, DiPerna DM, Sirianni RW (2020) Targeting small molecule delivery to the brain and spinal cord via intranasal administration of rabies virus glycoprotein (RVG29)-modified PLGA nanoparticles. Pharmaceutics 12. https://doi.org/10.3390/pharmaceutics12020093
Colton CA, Wilcock DM (2010) Assessing activation states in microglia. CNS Neurol Disord-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 9:174–191
Costa C, Moreira J, Amaral M, Lobo JS, Silva ACJJOCR (2019) Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J Control Release 295:187–200
Costa CP, Moreira JN, Sousa Lobo JM, Silva AC (2021) Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: a current overview of in vivo studies. Acta Pharmaceutica Sinica B 11:925–940. https://doi.org/10.1016/j.apsb.2021.02.012
Dai H et al (2010) Intrinsic targeting of inflammatory cells in the brain by polyamidoamine dendrimers upon subarachnoid administration. Nanomedicine 5:1317–1329
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V (2012) PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161:505–522
Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A (2016) Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol 44:381–391
De Jong WH, Borm PJ (2008) Drug delivery and nanoparticles:applications and hazards. Int J Nanomedicine 3:133–149. https://doi.org/10.2147/ijn.s596
Deirram N, Zhang C, Kermaniyan SS, Johnston APR, Such GK (2019) pH-responsive polymer nanoparticles for drug delivery. Macromol Rapid Commun 40:e1800917. https://doi.org/10.1002/marc.201800917
Dhuria SV, Hanson LR, Frey WH (2009) Novel vasoconstrictor formulation to enhance intranasal targeting of neuropeptide therapeutics to the central nervous system. J Pharmacol Exp Ther 328:312–320
Durand M et al (2001) Preliminary study of the deposition of aerosol in the maxillary sinuses using a plastinated model. J Aerosol Med 14:83–93
Elmowafy EM, Tiboni M, Soliman ME (2019) Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J Pharm Investig 49:347–380. https://doi.org/10.1007/s40005-019-00439-x
Elnaggar YSR, Etman SM, Abdelmonsif DA, Abdallah OY (2015) Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: optimization, biological efficacy, and potential toxicity. J Pharm Sci 104:3544–3556. https://doi.org/10.1002/jps.24557
English C, Aloi JJ (2015) New FDA-approved disease-modifying therapies for multiple sclerosis. Clin Ther 37:691–715
Erdő F, Bors LA, Farkas D, Bajza Á, Gizurarson S (2018) Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull 143:155–170. https://doi.org/10.1016/j.brainresbull.2018.10.009
Eskandari S, Varshosaz J, Minaiyan M, Tabbakhian M (2011) Brain delivery of valproic acid via intranasal administration of nanostructured lipid carriers: in vivo pharmacodynamic studies using rat electroshock model. Int J Nanomedicine 6:363–371. https://doi.org/10.2147/ijn.S15881
Fan Y, Chen M, Zhang J, Maincent P, Xia X, Wu W (2018) Updated progress of nanocarrier-based intranasal drug delivery systems for treatment of brain diseases. Crit Rev™ Therapeut Drug Carrier Syst 35
Farahavar G, Abolmaali SS, Gholijani N, Nejatollahi F (2019) Antibody-guided nanomedicines as novel breakthrough therapeutic, diagnostic and theranostic tools. Biomater Sci 7:4000–4016. https://doi.org/10.1039/c9bm00931k
Field P, Li Y, Raisman GJJON (2003) Ensheathment of the olfactory nerves in the adult rat. J Neurocytol 32:317–324
Froelich A, Osmałek T, Jadach B, Puri V, Michniak-Kohn B (2021) Microemulsion-based media in nose-to-brain drug delivery. Pharmaceutics 13:201
Furubayashi T et al (2007) Kinetic model to predict the absorption of nasally applied drugs from in vitro transcellular permeability of drugs. Biol Pharm Bull 30:1007–1010. https://doi.org/10.1248/bpb.30.1007
Gänger S, Schindowski K (2018) Tailoring formulations for intranasal nose-to-brain delivery: a review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics 10:116. https://doi.org/10.3390/pharmaceutics10030116
Gao M, Shen X, Mao S (2020) Factors influencing drug deposition in thenasal cavity upon delivery via nasal sprays. J Pharm Investig 50:251–259. https://doi.org/10.1007/s40005-020-00482-z
Gendelman HE et al (2015) Nanoneuromedicines for degenerative, inflammatory, and infectious nervous system diseases. Nanomedicine: Nanotechnology. Biol Med 11:751–767
Ghadiri M, Young PM, Traini DJP (2019) Strategies to Enhance Drug Absorption via Nasal and Pulmonary Routes. Pharmaceutics 11(3):113
Gilhus NE, Deuschl G (2019) Neuroinflammation—a common thread in neurological disorders. Nat Rev Neurol 15:429–430
Gitler AD, Dhillon P, Shorter J (2017) Neurodegenerative disease: models, mechanisms, and a new hope. The Company of Biologists Ltd
Gizurarson S (1993) The relevance of nasal physiology to the design of drug absorption studies. Adv Drug Deliv Rev 11:329–347. https://doi.org/10.1016/0169-409X(93)90015-V
González LF, Acuña E, Arellano G, Morales P, Sotomayor P, Oyarzun-Ampuero F, Naves R (2021) Intranasal delivery of interferon-β-loaded nanoparticles induces control of neuroinflammation in a preclinical model of multiple sclerosis: a promising simple, effective, non-invasive, and low-cost therapy. J Control Release 331:443–459. https://doi.org/10.1016/j.jconrel.2020.11.019
Graff CL, Pollack GM (2003) P-Glycoprotein attenuates brain uptake of substrates after nasal instillation. Pharm Res 20:1225–1230
Greish K (2010) Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. In: Cancer nanotechnology. Springer, pp 25–37
Guo J, Jiang X, Gui S (2016) RNA interference-based nanosystems for inflammatory bowel disease therapy. Int J Nanomed 11:5287
Gutiérrez J, González C, Maestro A, Solè I, Pey C, Nolla J (2008) Nano-emulsions: new applications and optimization of their preparation. Curr Opin Colloid Interface Sci 13:245–251
Guzman-Martinez L, Maccioni RB, Andrade V, Navarrete LP, Pastor MG, Ramos-Escobar N (2019) Neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol 10:1008
Han H et al (2019) Monocytes as carriers of magnetic nanoparticles for tracking inflammation in the epileptic rat brain. Curr Drug Deliv 16:637–644. https://doi.org/10.2174/1567201816666190619122456
Hernando S, Herran E, Figueiro-Silva J, Pedraz JL, Igartua M, Carro E, Hernandez RM (2018) Intranasal administration of TAT-conjugated lipid nanocarriers loading GDNF for Parkinson’s disease. Mol Neurobiol 55:145–155. https://doi.org/10.1007/s12035-017-0728-7
Hoyos-Ceballos GP et al (2020) PLGA-PEG-ANG-2 nanoparticles for blood-brain barrier crossing: proof-of-concept study. Pharmaceutics 12. https://doi.org/10.3390/pharmaceutics12010072
Huart C, Rombaux P, Hummel TJJOB, biomembranes, (2019) Neural plasticity in developing and adult olfactory pathways–focus on the human olfactory bulb. J Bioenerg Biomembr 51:77–87
Ibrahim Bekraki A (2020) Chapter 7 - Liposomes-and niosomes-based drug delivery systems for tuberculosis treatment. In: Kesharwani P (ed) Nanotechnology based approaches for tuberculosis treatment. Academic Press, pp 107–122. https://doi.org/10.1016/B978-0-12-819811-7.00007-2
Islam SU, Shehzad A, Ahmed MB, Lee YS (2020) Intranasal delivery of nanoformulations: a potential way of treatment for neurological disorders. Molecules 25:1929
Jeong SJ et al (2017a) Intravenous immune-modifying nanoparticles as a therapy for spinal cord injury in mice. Neurobiol Dis 108:73–82
Johnson NJ, Hanson LR, Frey WHJMP (2010) Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol Pharm 7:884–893
Juthani R et al (2020) Ultrasmall core-shell silica nanoparticles for precision drug delivery in a high-grade malignant brain tumor model. Clin Cancer Res 26:147–158. https://doi.org/10.1158/1078-0432.Ccr-19-1834
Karasulu HY (2008) Microemulsions as novel drug carriers: the formation, stability, applications and toxicity. Expert Opin Drug Deliv 5:119–135. https://doi.org/10.1517/17425247.5.1.119
Kaur N, Chugh H, Sakharkar MK, Dhawan U, Chidambaram SB, Chandra R (2020) Neuroinflammation mechanisms and phytotherapeutic intervention: a systematic review. ACS Chem Neurosci 11:3707–3731
Keller L-A, Merkel O, Popp A (2021) Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Deliv Transl Res. https://doi.org/10.1007/s13346-020-00891-5
Keller L-A, Merkel O, Popp AJDD, Research T (2021b) Intranasal drug delivery: opportunities and toxicologic challenges during drug development.1–23
Khallaf RA, Aboud HM, Sayed OM (2020) Surface modified niosomes of olanzapine for brain targeting via nasal route; preparation, optimization, and in vivo evaluation. J Liposome Res 30:163–173. https://doi.org/10.1080/08982104.2019.1610435
Khongkow M, Yata T, Boonrungsiman S, Ruktanonchai UR, Graham D, Namdee K (2019) Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood–brain barrier penetration. Sci Rep 9:8278. https://doi.org/10.1038/s41598-019-44569-6
Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW (2014) Pattern recognition receptors and central nervous system repair. Exp Neurol 258:5–16
Kim D, Shin K, Kwon SG, Hyeon T (2018) Synthesis and biomedical applications of multifunctional nanoparticles. Adv Mater 30:e1802309. https://doi.org/10.1002/adma.201802309
Knight DA, Holgate STJR (2003) The Airway Epithelium: Structural and Functional Properties in Health and Disease. Respirology 8:432–446
Koenigsknecht-Talboo J, Landreth GE (2005) Microglial phagocytosis induced by fibrillar β-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci 25:8240–8249
Kozlovskaya L, Abou-Kaoud M, Stepensky DJJOCR (2014) Quantitative analysis of drug delivery to the brain via nasal route. J Control Release 189:133–140
Kreuter J (2014) Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev 71:2–14
Kulkarni SA, Feng SS (2011) Effects of surface modification on delivery efficiency of biodegradable nanoparticles across the blood-brain barrier. Nanomedicine (Lond) 6:377–394. https://doi.org/10.2217/nnm.10.131
Kumar A, Pandey AN, Jain SKJDd (2016a) Nasal-nanotechnology: revolution for efficient therapeutics delivery. 23:671–683
Kumar H, Mishra G, Sharma AK, Gothwal A, Kesharwani P, Gupta U (2017) Intranasal drug delivery: a non-invasive approach for the better delivery of neurotherapeutics. Pharm Nanotechnol 5:203–214. https://doi.org/10.2174/2211738505666170515113936
Kumar M, Misra A, Babbar AK, Mishra AK, Mishra P, Pathak K (2008a) Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int J Pharm 358:285–291. https://doi.org/10.1016/j.ijpharm.2008.03.029
Kumar M, Misra A, Mishra AK, Mishra P, Pathak K (2008b) Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J Drug Target 16:806–814. https://doi.org/10.1080/10611860802476504
Kumar M, Sharma P, Maheshwari R, Tekade M, Shrivastava SK, Tekade RK (2018) Chapter 15 - Beyond the blood–brain barrier: facing new challenges and prospects of nanotechnology-mediated targeted delivery to the brain. In: Kesharwani P, Gupta U (eds) Nanotechnology-based targeted drug delivery systems for brain tumors. Academic Press, pp 397–437. https://doi.org/10.1016/B978-0-12-812218-1.00015-4
Kumar NN, Gautam M, Lochhead JJ, Wolak DJ, Ithapu V, Singh V, Thorne RGJSR (2016b) Relative vascular permeability and vascularity across different regions of the rat nasal mucosa: implications for nasal physiology and drug delivery. 6:1-14
Kumar R, Aadil KR, Ranjan S, Kumar VB (2020) Advances in nanotechnology and nanomaterials based strategies for neural tissue engineering. J Drug Deliv Sci Technol 57:101617
Lavoie J et al (2017) The Olfactory Neural Epithelium as a Tool in Neuroscience. Trends Mol Med 23:100–103
Li Y, Hu X, Liu Y, Bao Y, An L (2009) Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology 56:580–589. https://doi.org/10.1016/j.neuropharm.2008.10.016
Liddelow SA et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487
Lochhead JJ, Thorne RG (2012) Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev 64:614–628
Lochhead JJ, Thorne RG (2014) Intranasal drug delivery to the brain. In: Drug delivery to the brain. Springer, pp 401–431
Maccioni RB, Rojo LE, Fernandez JA, Kuljis RO (2009) The role of neuroimmunomodulation in Alzheimer’s disease. Ann N Y Acad Sci 1153:240–246
Madaan K, Kumar S, Poonia N, Lather V, Pandita D (2014) Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci 6:139
Malam Y, Loizidou M, Seifalian AM (2009) Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci 30:592–599
Marttin E, Schipper NG, Verhoef JC, Merkus FWJA (1998) Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev 29:13–38
Masserini M (2013) Nanoparticles for brain drug delivery. Int Scholar Res Notices 2013
Meda L, Baron P, Scarlato G (2001) Glial activation in Alzheimer’s disease: the role of Aβ and its associated proteins. Neurobiol Aging 22:885–893
Meng Q et al (2018) Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int J Nanomedicine 13:705–718. https://doi.org/10.2147/ijn.S151474
Mishra MK et al (2014) Dendrimer brain uptake and targeted therapy for brain injury in a large animal model of hypothermic circulatory arrest. ACS Nano 8:2134–2147
Mistry A, Stolnik S, Illum L (2009) Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm 379:146–157. https://doi.org/10.1016/j.ijpharm.2009.06.019
Moeinzadeh S, Jabbari E (2017) Nanoparticles and their applications. In: Springer handbook of nanotechnology. Springer, pp 335–361
Müller RH, Mäder K, Gohla S (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm 50:161–177
Musumeci T et al (2018) Oxcarbazepine free or loaded PLGA nanoparticles as effective intranasal approach to control epileptic seizures in rodents. Eur J Pharm Biopharm 133:309–320. https://doi.org/10.1016/j.ejpb.2018.11.002
Neha B, Ganesh B, Preeti K (2013) Drug delivery to the brain using polymeric nanoparticles: a review. Int J Pharmaceut Life Sci 2:107–132
Newman SP, Pitcairn GR, Dalby RN (2004) Drug delivery to the nasal cavity: in vitro and in vivo assessment. Crit Rev™ Therapeut Drug Carrier Syst 21
Oliveira P, Fortuna A, Alves G, Falcao A (2016) Drug-metabolizing enzymes and efflux transporters in nasal epithelium: influence on the bioavailability of intranasally administered drugs. Curr Drug Metab 17:628–647
Papa S et al (2016) Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials 75:13–24
Patra JK et al (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16:1–33
Peviani M, Palmiero UC, Cecere F, Milazzo R, Moscatelli D, Biffi A (2019) Biodegradable polymeric nanoparticles administered in the cerebrospinal fluid: brain biodistribution, preferential internalization in microglia and implications for cell-selective drug release. Biomaterials 209:25–40
Raj R, Wairkar S, Sridhar V, Gaud R (2018) Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: development, characterization and in vivo anti-Parkinson activity. Int J Biol Macromol 109:27–35. https://doi.org/10.1016/j.ijbiomac.2017.12.056
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353:777–783
Rassu G, Soddu E, Cossu M, Gavini E, Giunchedi P, Dalpiaz A (2016) Particulate formulations based on chitosan for nose-to-brain delivery of drugs. A review. J Drug Deliv Sci Technol 32:77–87
Rinaldi F et al (2019) inPentasomes: an innovative nose-to-brain pentamidine delivery blunts MPTP parkinsonism in mice. J Control Release 294:17–26
Roco MC, Mirkin CA, Hersam MC (2011) Nanotechnology research directions for societal needs in 2020: summary of international study. J Nanopart Res 13:897–919. https://doi.org/10.1007/s11051-011-0275-5
Sabir F, Ismail R, Csoka IJD (2020) Nose-to-brain delivery of antiglioblastoma drugs embedded into lipid nanocarrier systems: status quo and outlook. Drug Discov Today 25:185–194
Saha S et al (2020) Amphetamine decorated cationic lipid nanoparticles cross the blood-brain barrier: therapeutic promise for combating glioblastoma. J Mater Chem B 8:4318–4330. https://doi.org/10.1039/c9tb02700a
Schipper NG, Verhoef JC, Merkus FWJPr (1991) The nasal mucociliary clearance: relevance to nasal drug delivery. 8:807-814
Seju U, Kumar A, Sawant KK (2011) Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: in vitro and in vivo studies. Acta Biomater 7:4169–4176. https://doi.org/10.1016/j.actbio.2011.07.025
Selvaraj K, Gowthamarajan K, Karri VVSR (2018) Nose to brain transport pathways an overview: potential of nanostructured lipid carriers in nose to brain targeting. Artif Cells Nanomed Biotechnol 46:2088–2095
Selvaraj K, Gowthamarajan K, Karri VVSRJAc, nanomedicine, biotechnology (2018b) Nose to brain transport pathways an overview: potential of nanostructured lipid carriers in nose to brain targeting. 46:2088-2095
Shabab T, Khanabdali R, Moghadamtousi SZ, Kadir HA, Mohan G (2017) Neuroinflammation pathways: a general review. Int J Neurosci 127:624–633
Shah B, Khunt D, Misra M, Padh H (2016) Application of Box-Behnken design for optimization and development of quetiapine fumarate loaded chitosan nanoparticles for brain delivery via intranasal route*. Int J Biol Macromol 89:206–218. https://doi.org/10.1016/j.ijbiomac.2016.04.076
Shao Y, Peng H, Huang Q, Kong J, Xu H (2015) Quetiapine mitigates the neuroinflammation and oligodendrocyte loss in the brain of C57BL/6 mouse following cuprizone exposure for one week. Eur J Pharmacol 765:249–257. https://doi.org/10.1016/j.ejphar.2015.08.046
Shastri A, Bonifati DM, Kishore U (2013) Innate immunity and neuroinflammation. Mediators Inflamm 2013
Sochocka M, Diniz BS, Leszek J (2017) Inflammatory response in the CNS: friend or foe? Mol Neurobiol 54:8071–8089
Su Y et al (2020) Intranasal delivery of targeted nanoparticles loaded with mir-132 to brain for the treatment of neurodegenerative diseases. Front Pharmacol 11:1165
Thorne R, Pronk G, Padmanabhan V, Frey Ii WJN (2004) Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. 127:481-496
Tilleux S, Hermans E (2007) Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res 85:2059–2070
Tiwari A, Mahadik KR, Gabhe SY (2020) Piperine: a comprehensive review of methods of isolation, purification, and biological properties. Med Drug Discov 7:100027. https://doi.org/10.1016/j.medidd.2020.100027
Tomalia DA, Nixon LS, Hedstrand DM (2020) The role of branch cell symmetry and other critical nanoscale design parameters in the determination of dendrimer encapsulation properties. Biomolecules 10:642
Torchilin VP (2007) Micellar nanocarriers: pharmaceutical perspectives. Pharm Res 24:1–16
Trotta V et al (2018) Brain targeting of resveratrol by nasal administration of chitosan-coated lipid microparticles. Eur J Pharm Biopharm 127:250–259. https://doi.org/10.1016/j.ejpb.2018.02.010
Tzeng SY, Green JJ (2013) Therapeutic nanomedicine for brain cancer. Ther Deliv 4:687–704
Ud Din F, Aman W, Ullah I, Qureshi OS, Mustapha O, Shafique S, Zeb A (2017) Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int J Nanomed 12:7291
Veronesi MC, Alhamami M, Miedema SB, Yun Y, Ruiz-Cardozo M, Vannier MW (2020) Imaging of intranasal drug delivery to the brain. Am J Nucl Med Mol Imaging 10:1
Vyas TK, Babbar AK, Sharma RK, Misra A (2005) Intranasal mucoadhesive microemulsions of zolmitriptan: preliminary studies on brain-targeting. J Drug Target 13:317–324. https://doi.org/10.1080/10611860500246217
Vyas TK, Babbar AK, Sharma RK, Singh S, Misra A (2006a) Intranasal mucoadhesive microemulsions of clonazepam: preliminary studies on brain targeting. J Pharm Sci 95:570–580. https://doi.org/10.1002/jps.20480
Vyas TK, Babbar AK, Sharma RK, Singh S, Misra A (2006b) Preliminary brain-targeting studies on intranasal mucoadhesive microemulsions of sumatriptan. AAPS PharmSciTech 7:E49-e57. https://doi.org/10.1208/pt070108
Wang K et al. (2020) Therapeutic nanomaterials for neurological diseases and cancer therapy. J Nanomater 2020
Wen Z et al (2011) Odorranalectin-conjugated nanoparticles: preparation, brain delivery and pharmacodynamic study on Parkinson’s disease following intranasal administration. J Control Release 151:131–138. https://doi.org/10.1016/j.jconrel.2011.02.022
Wolburg H, Wolburg-Buchholz K, Sam H, Horvát S, Deli MA, Mack AF (2008) Epithelial and endothelial barriers in the olfactory region of the nasal cavity of the rat. Histochem Cell Biol 130:127–140
Yadav S, Gandham SK, Panicucci R, Amiji MM (2016) Intranasal brain delivery of cationic nanoemulsion-encapsulated TNFα siRNA in prevention of experimental neuroinflammation. Nanomedicine 12:987–1002. https://doi.org/10.1016/j.nano.2015.12.374
Yao M et al (2020) Engineering of SPECT/photoacoustic imaging/antioxidative stress triple-function nanoprobe for advanced mesenchymal stem cell therapy of cerebral ischemia. ACS Appl Mater Interfaces 12:37885–37895. https://doi.org/10.1021/acsami.0c10500
Yildirimer L, Thanh NTK, Loizidou M, Seifalian AM (2011) Toxicology and clinical potential of nanoparticles. Nano Today 6:585–607. https://doi.org/10.1016/j.nantod.2011.10.001
Zhang C et al (2014) Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer’s disease. Int J Pharm 461:192–202. https://doi.org/10.1016/j.ijpharm.2013.11.049
Zhang F, Lin Y-A, Kannan S, Kannan RM (2016a) Targeting specific cells in the brain with nanomedicines for CNS therapies. J Control Release 240:212–226
Zhang Q, Jiang X, Jiang W, Lu W, Su L, Shi Z (2004) Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain. Int J Pharm 275:85–96. https://doi.org/10.1016/j.ijpharm.2004.01.039
Zhang T-T, Li W, Meng G, Wang P, Liao W (2016b) Strategies for transporting nanoparticles across the blood–brain barrier. Biomater Sci 4:219–229
Zielińska A et al (2020) Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules 25:3731
Author information
Authors and Affiliations
Contributions
FM and ND conceived of the presented idea. FM and ND equally participated in drafting the article. FM participated in revising it critically for important intellectual content. FM and ND gave final approval of the version to be submitted and any revised version.
Corresponding author
Ethics declarations
Ethical approval
N/A
Consent to participate
N/A
Consent to publish
N/A
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moradi, F., Dashti, N. Targeting neuroinflammation by intranasal delivery of nanoparticles in neurological diseases: a comprehensive review. Naunyn-Schmiedeberg's Arch Pharmacol 395, 133–148 (2022). https://doi.org/10.1007/s00210-021-02196-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-021-02196-x