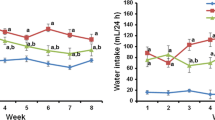
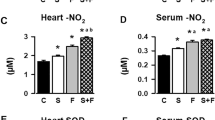
Abstract
Pregnancy is an insulin-resistant condition especially at near term predisposing maternal kidneys to hyperinsulinemia-induced oxidative stress. The impact of fructose on renal metabolic dysregulation and oxidative stress in pregnancy requires elucidation. Short-chain fatty acids (SCFAs) are known for protective roles in oxidative stress conditions. Therefore, the study aimed at investigating fructose-induced glucose dysregulation and renal oxidative stress in pregnant and non-pregnant rats and the possible preventive role of SCFA, acetate. Thirty female Wistar rats were grouped (n = 5/group). Three groups were made pregnant (P); the other three remained non-pregnant (NP). Both pregnant and non-pregnant rats received drinking water (control), 10% fructose (w/v) (NP+F or P+F), and 10% (w/v) fructose plus sodium acetate (200 mg/kg) (NP+F+A or P+F+A) for 3 weeks. Renal and plasma glutathione antioxidant index (GSH/GSSG), G6PDH, and adenosine were significantly lower in NP+F and P+F groups compared with control while renal and plasma adenosine deaminase (ADA), xanthine oxidase (XO), uric acid (UA), lactate dehydrogenase (LDH), and malonaldehyde (MDA) were significantly elevated in NP+F and P+F groups compared with controls. HOMA-IR showed marked impairment in both NP+F and P+F groups. The P+F group revealed greater suppression in plasma and renal G6PDH-dependent antioxidant index, adenosine, and aggravation of LDH, MDA compared with the NP+F group (p < 0.05). Sodium acetate reduces plasma and renal surrogate oxidative stress markers, improved G6PD-dependent antioxidant index, and HOMA-IR in NP+F and P+F groups. Pregnancy exacerbates fructose-induced insulin resistance and renal oxidative stress whereas acetate ameliorated fructose-induced redox and glucose dysregulation in pregnant and non-pregnant rats.






Similar content being viewed by others
Data availability
Data for the research work were made available at submission.
References
Adachi K, Sugiyama T, Yamaguchi Y, Tamura Y, Izawa S, Hijikata Y, Ebi M, Funaki Y, Ogasawara N, Goto C et al (2019) Gut microbiota disorders cause type 2 diabetes mellitus and homeostatic disturbances ingut-related metabolism in Japanese subjects. J Clin Biochem Nutr 64:231–238
Ayala A, Munoz MF, Arguelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med Cell Longev 2014:360438
Beutler E (1984) Red Cell Metabolism: A Manual of Biochemical Methods, 3rd edn. Grune & Strattan, New York
Bray GA, Nielsen SJ, Popkin BM (2004) Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Nutr 79:537–543
Caliceti C, Calabria D, Roda A, Cicero AF (2017) Fructose Intake, Serum Uric Acid, and cardiometabolic Disorders: A Critical Review. Nutrients 9:395. https://doi.org/10.3390/nu9040395 [Google Scholar]
Cerf ME (2013) Beta cell dysfunction and insulin resistance. Front Endocrinol 4:37 1–12
Choi Y, Abdelmegeed M, Song BJ (2017) Diet high in fructose promotes liver steatosis and hepatocyte apoptosis in C57BL/6J female mice: role of disturbed lipid homeostasis and increased oxidative stress. Food Chem Toxicol 103:111–121
Dharmalingam M, Yamasandhi PG (2018) Nonalcoholic fatty liver disease and type 2 diabetes mellitus. Indian J Endocrinol Metabolism 22:421–428
Fabiane R, Leidiane D, Walter SN, Thissiane D (2018) Influence of gestational diabetes on the activity of δ-aminolevulinate dehydratase and oxidative stress biomarkers. Redox Rep 23(1):63–67. https://doi.org/10.1080/13510002.2017.1402981
Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, Carling D, Swann JR, Gibson G, Viardot A, Morrison D, Louise Thomas E, Bell JD (2014) The shortchain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 5:3611
Jadhav AA, Jain A (2013) Adenosine deaminase activity in normal pregnancy and pregnancy associated disorders. Arch PhysiolBiochem 119:88–91
Kelley EE, Khoo NK, Hundley NJ, Malik UZ, Freeman BA, Tarpey MM (2010) Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med 48(4):493–498
Lee S, Park H, Ki Y, Lee H, Han MS (2019) Development of a Simple Assay Method for Adenosine Deaminase via Enzymatic Formation of an Inosine-Tb3+ Complex. Sensors (Basel) 19(12):E2728. https://doi.org/10.3390/s19122728
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 4(8):118–126. https://doi.org/10.4103/0973-7847.70902]
Lu F, Chauhan AK, Fernandes SM, Walsh MT, Wagner DD, Davis AE III (2008) The effect of C1 inhibitor on intestinal ischemia and reperfusion injury. Am J Physiol Gastrointest Liver Physiol 295(5):G1042–G1049
Madlala HP, Maarman GJ, Ojuka E (2016) Uric acid and transforming growth factor in fructose-induced production of reactive oxygen species in skeletal muscle. Nutr Rev 74:259–266
Mandal AK, Mount DB (2015) The molecular physiology of uric acid homeostasis. Annu Rev Physiol 77:323–345
Maslowki KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D et al (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 461:1282–1286
Meghri K, Maria F, Penelope DH (2019) Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxidative Med Cell Longev 1279250:29. https://doi.org/10.1155/2019/1279250
Moriyama M, Kurebayashi R, Kawabe K, Takano K, Nakamura Y (2016) Acetate attenuates lipopolysaccharide-induced nitric oxide production through an anti-oxidative mechanism in cultured primary rat astrocytes. Neurochem Res 41:3138–3146
Mortensen PB, Clausen MR (1996) Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol Suppl 216:132–148
Napso T, Yong HE, Lopez-Tello J, Sferruzzi-Perri AN (2018) The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front Physiol 9:1091
Rahman I, Kode A, Biswas SK (2007) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1(6):3159–3165
Réus GZ, Carlessi AS, Silva RH, Ceretta LB, Quevedo J (2019) Relationship of oxidative stress as a link between diabetes mellitus and major depressive disorder. Oxidative Med Cell Longev 2019:1–6. https://doi.org/10.1155/2019/8637970
Schiavone S, Camerino GM, Mhillaj E, Zotti M, Colaianna M, De Giorgi A, Trotta A, Cantatore FP, Conte E, Bove M et al (2017) Visceral fat dysfunctions in the rat social isolation model of psychosis. Front Pharmacol 8:87
Uchiyama M, Mihars M (1978) Determination of malondialdehyde precursor in tissues by thiobarbituric acid. Ann Biochem 86:271–278
Volodymyr IL (2012) Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 736837:26. https://doi.org/10.1155/2012/736837
Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM (2008) Dietary fructose consumption among US children and adults: The Third National Health and Nutrition Examination Survey. Medscape J Med 10:160
Warmbrunn MV, Herrema H, Aron-Wisnewsky J, Soeters MR, Van Raalte DH, Nieuwdorp M (2020) Gut microbiota: a promising target against cardiometabolic diseases. Expert Rev Endocrinol Metab 15(1):13–27. https://doi.org/10.1080/17446651.2020.1720511
Wong JM, de Souza R, Kendall WC, Emam A, Jenkins DJ (2006) Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40(3):235–243. https://doi.org/10.1097/00004836-200603000-00015
Xu Y, Wang Y, Yan S, Yang Q, Zhou Y, Zeng X et al (2017) Regulation of endothelial intracellular adenosine via adenosine kinase epigenetically modulates vascular inflammation. Nat Commun 16:8–943
Ye J (2013) Improving insulin sensitivity with HDAC inhibitor. Diabetes 62:685–687
Zargaraan A, Kamaliroosta L, Yaghoubi AS, Mirmoghtadaie L (2016) Effect of substitution of sugar by high fructose corn syrup on the physicochemical properties of bakery and dairy products: a review. Nutr Food Sci Res 3(4):3–11
Zhang Y, Handy DE, Loscalzo J (2005) Adenosine-dependent induction of glutathione peroxidase 1 in human primary endothelia cells and protection against oxidative stress. Circ Res 96:83
Zhao F, Liu Q, Cao J, Xu Y, Pei Z, Fan H, Yuan Y, Shen X, Li C (2019) A sea cucumber (Holothurialeucospilota) polysaccharide improves the gut microbiome to alleviate the symptoms of type 2 diabetes mellitus in Goto-Kakizaki rats. Food Chem Toxicol 135:110886. https://doi.org/10.1016/j.fct.2019.110886
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. PhysiologicalReviews. 94(3):909–950
Acknowledgements
The authors acknowledge the technical support of HOPE Cardiometabolic Research Team and Mr. Adebowale of Bridge Biotech is also appreciated for the laboratory analysis.
Funding
There was no external source of funding for the work.
Author information
Authors and Affiliations
Contributions
AOO, OSM, and LAO conceived and designed the research. AOO, EDA, and SBS conducted the experiments. AOO, OSM, EDA, SBS, and LAO contributed to the new reagents and analytical kits. AOO, OSM, EDA, and LAO analyzed and interpreted the data. AOO, OSM, EDA, SBS, and LAO drafted the manuscript. AOO, OSM, EDA, SBS, and LAO. All authors read and approved the manuscript, and all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the work was obtained from the University of Ilorin Ethical Review Committee with protocol number UERC/ASN/2016/357, having passed the Faculty of Basic Medical Sciences, University of Ilorin Ethical Review Committee. The investigation was conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Review Board of the University of Ilorin, with protocol identification number UERC/ASN/2016/357, and every effort was made to minimize both the number of animals used and their sufferings.
The following underlisted authors agreed and freely participated in the review of this work and made a sizeable contribution to the progress of the work.
Adewumi Oluwafemi Oyabambi, oyabambi.ao@unilorin.edu.ng
Olugbenga Samuel Micheal, michaelolugbenga2@gmail.com
Emmanuel Damilare Areola, areola577@gmail.com
Salam Babatunde Saliu, salambat50@gmail.com
Lawrence Aderemi Olatunji, tunjilaw@unilorin.edu.ng
Consent for publication
The following underlisted authors agreed that the manuscript titled “Sodium acetate ameliorated systemic and renal oxidative stress in high-fructose insulin-resistant pregnant Wistar rats.” be published within your Journal, Naunyn-Schmiedeberg's Archives of Pharmacology.
Adewumi Oluwafemi Oyabambi, oyabambi.ao@unilorin.edu.ng
Olugbenga Samuel Micheal, michaelolugbenga2@gmail.com
Emmanuel Damilare Areola, areola577@gmail.com
Salam Babatunde Saliu, salambat50@gmail.com
Lawrence Aderemi Olatunji, tunjilaw@unilorin.edu.ng
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Oyabambi, A.O., Michael, O.S., Areola, E.D. et al. Sodium acetate ameliorated systemic and renal oxidative stress in high-fructose insulin-resistant pregnant Wistar rats. Naunyn-Schmiedeberg's Arch Pharmacol 394, 1425–1435 (2021). https://doi.org/10.1007/s00210-021-02058-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-021-02058-6