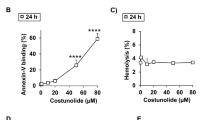
Abstract
In recent years, there have been efforts to develop therapeutic agents that target metabolic enzyme systems in addition to existing treatment in possible cancer treatments. Cyclophosphamide (CYP) is an anticancer drug commonly used in various cancer treatments. Chrysin (CH) and naringin (NR) are natural flavonoids that possess many medicinal and pharmacological properties. In the present study, we aimed to investigate the effect of CH and NR against CYP-induced toxicity on some metabolic enzyme activities. For this purpose, 56 male rats were randomly divided into 8 groups in our in vivo study. The rats were pretreated with CH (25 and 50 mg/kg bw) and NR (50 and 100 mg/kg bw) for 7 days before administering a single dose of CYP (200 mg/kg bw) on the seventh day. According to the in vivo results of our study, it was observed that CH and NR regulated abnormal changes in CYP-induced enzyme activities. In addition, our in vitro study, G6PD enzyme was purified from rat erythrocyte using affinity chromatography. The effects of CH, NR, and CYP were investigated on the purified enzyme. It was determined that CH increased the enzyme activity, CYP ineffective on the enzyme activity, whereas NR inhibited the enzyme activity noncompetitively.

Graphical abstract






Similar content being viewed by others
References
Adem S, Ciftci M (2016) Purification and biochemical characterization of glucose 6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase and glutathione reductase from rat lung and inhibition effects of some antibiotics. J Enzyme Inhib Med Chem 6366:1–7. https://doi.org/10.3109/14756366.2015.1132711
Akkemik E, Şentürk M, Özgerİş FB et al (2011) In vitro eff ects of some drugs on human erythrocyte glutathione reductase. 41:235–241. https://doi.org/10.3906/sag-1002-4
Arnér ESJ, Holmgren A (2006) The thioredoxin system in cancer. Semin Cancer Biol 16:420–426. https://doi.org/10.1016/j.semcancer.2006.10.009
Balendiran GK, Dabur R, Fraser D (2004) The role of glutathione in cancer. Cell Biochem Funct 22:343–352. https://doi.org/10.1002/cbf.1149
Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL (2009) Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem 390:191–214. https://doi.org/10.1515/BC.2009.033
Bayindir S, Temel Y, Ayna A, Ciftci M (2018a) The synthesis of N-benzoylindoles as inhibitors of rat erythrocyte glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase. J Biochem Mol Toxicol 32:1–9. https://doi.org/10.1002/jbt.22193
Bayindir S, Ayna A, Temel Y, Çiftci M (2018b) The synthesis of new oxindoles as analogs of natural product 3,3′-bis(indolyl)oxindole and in vitro evaluation of the enzyme activity of G6PD and 6PGD. Turkish J Chem 42. https://doi.org/10.3906/kim-1706-51
Bayramoğlu Akkoyun M, Bengü AŞ, Temel Y, Akkoyun HT, Ekin S, Ciftci M (2018) The effect of astaxanthin and cadmium on rat erythrocyte G6PD, 6PGD, GR, and TrxR enzymes activities in vivo and on rat erythrocyte 6PGD enzyme activity in vitro. J Biochem Mol Toxicol 32:1–5. https://doi.org/10.1002/jbt.22170
Beydemir S, Gülçin I, Küfrevioğlu OI, Ciftçi M (2003) Glucose 6-phosphate dehydrogenase: in vitro and in vivo effects of dantrolene sodium. Pol J Pharmacol 55:787–792
Beydemir GI, Hisar O et al (2005) Effect of melatonin on glueose-6-phosphate dehydrogenase from rainbow trout (Oncorhynchus my kiss) erythrocytes in vitro and in vivo. J Appl Anim Res 28:65–68. https://doi.org/10.1080/09712119.2005.9706791
Branco V, Godinho-santos A, Gonçalves J et al (2014) Free radical biology and medicine mitochondrial thioredoxin reductase inhibition, selenium status , and Nrf-2 activation are determinant factors modulating the toxicity of mercury compounds. Free Radic Biol Med 73:95–105. https://doi.org/10.1016/j.freeradbiomed.2014.04.030
Caglayan C, Temel Y, Kandemir FM, Yildirim S, Kucukler S (2018) Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ Sci Pollut Res 25:20968–20984. https://doi.org/10.1007/s11356-018-2242-5
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11:85–95. https://doi.org/10.1038/nrc2981
Ceylan M, Kocyigit UM, Usta NC, Gürbüzlü B, Temel Y, Alwasel SH, Gülçin İ (2016) Synthesis, carbonic anhydrase I and II isoenzymes inhibition properties, and antibacterial activities of novel tetralone-based 1,4-benzothiazepine derivatives. J Biochem Mol Toxicol 31. https://doi.org/10.1002/jbt.21872
Reviews B, Deluca VA (1975) Fibre-optic endoscopy. 310–311
Eldutar E, Kandemir FM, Kucukler S, Caglayan C (2017) Restorative effects of Chrysin pretreatment on oxidant–antioxidant status, inflammatory cytokine production, and apoptotic and autophagic markers in acute paracetamol-induced hepatotoxicity in rats: an experimental and biochemical study. J Biochem Mol Toxicol 31:4–9. https://doi.org/10.1002/jbt.21960
Gülçin I (2012) Antioxidant activity of food constituents: an overview. Arch Toxicol 86:345–391. https://doi.org/10.1007/s00204-011-0774-2
Gulcin İ (2020) Antioxidants and antioxidant methods: an updated overview
Habibi E, Shokrzadeh M, Chabra A, Naghshvar F, Keshavarz-Maleki R, Ahmadi A (2015) Protective effects of Origanum vulgare ethanol extract against cyclophosphamide-induced liver toxicity in mice. Pharm Biol 53:10–15. https://doi.org/10.3109/13880209.2014.908399
Ibrahim MA, Ghazy AHM, Salem AMH, Ghazy MA, Abdel-Monsef MM (2015) Biochemical characterization of buffalo liver glucose-6-phosphate dehydrogenase isoforms. Protein J 34:193–204. https://doi.org/10.1007/s10930-015-9615-0
Jakoby B (1974) A novel strate. 249:7140–7149
Kandemir FM, Kucukler S, Caglayan C et al (2017) Therapeutic effects of silymarin and naringin on methotrexate-induced nephrotoxicity in rats: biochemical evaluation of anti-inflammatory, antiapoptotic, and antiautophagic properties. J Food Biochem 41. https://doi.org/10.1111/jfbc.12398
Kern JC, Kehrer JP (2002) Acrolein-induced cell death: a caspase-influenced decision between apoptosis and oncosis/necrosis. Chem Biol Interact 139:79–95. https://doi.org/10.1016/S0009-2797(01)00295-2
Kocyigit UM, Aslan ON, Gulcin I, Temel Y, Ceylan M (2016) Synthesis and carbonic anhydrase inhibition of novel 2-(4-(aryl)thiazole-2-yl)-3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3(2H)-dione derivatives. Arch Pharm (Weinheim) 349:955–963. https://doi.org/10.1002/ardp.201600092
Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J (2020) Flavonoids as anticancer agents. Nutrients 12:1–25. https://doi.org/10.3390/nu12020457
Köse LP, Gülçin I, Gören AC, Namiesnik J, Martinez-Ayala AL, Gorinstein S (2015) LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind Crop Prod 74:712–721. https://doi.org/10.1016/j.indcrop.2015.05.034
Liao S, Williams-Ashman HG (1964) Glutathione reductase. Enzyme 13:888–894. https://doi.org/10.1007/978-3-662-11689-0_30
Lin R, Elf S, Shan C, et al (2015) 6-Phosphogluconate dehydrogenase links oxidative PPP , lipogenesis and tumour growth by inhibiting LKB1 – AMPK signalling. 17:. https://doi.org/10.1038/ncb3255
Motawi TMK, Sadik NAH, Refaat A (2010) Cytoprotective effects of DL-alpha-lipoic acid or squalene on cyclophosphamide-induced oxidative injury: an experimental study on rat myocardium , testicles and urinary bladder. Food Chem Toxicol 48:2326–2336. https://doi.org/10.1016/j.fct.2010.05.067
Nathan C, Ding A (2010) Snapshot: reactive oxygen intermediates (ROI). Cell 140:8–10. https://doi.org/10.1016/j.cell.2010.03.008
Naz S, Imran M, Rauf A, Orhan IE, Shariati MA, Iahtisham-Ul-Haq, IqraYasmin, Shahbaz M, Qaisrani TB, Shah ZA, Plygun S, Heydari M (2019) Chrysin: pharmacological and therapeutic properties. Life Sci 235:116797. https://doi.org/10.1016/j.lfs.2019.116797
Özmen I, Küfrevioǧlu ÖI (2004) Effects of antiemetic drugs on glucose 6-phosphate dehydrogenase and some antioxidant enzymes. Pharmacol Res 50:499–504. https://doi.org/10.1016/j.phrs.2004.05.007
Pereira RMS, Andrades NED, Paulino N, Sawaya A, Eberlin M, Marcucci M, Favero G, Novak E, Bydlowski S (2007) Synthesis and characterization of a metal complex containing naringin and cu, and its antioxidant, antimicrobial, antiinflammatory and tumor cell cytotoxicity. Molecules 12:1352–1366. https://doi.org/10.3390/12071352
Pljesa-Ercegovac M, Savic-Radojevic A, Matic M, Coric V, Djukic T, Radic T, Simic T (2018) Glutathione transferases: potential targets to overcome chemoresistance in solid tumors. Int J Mol Sci 19. https://doi.org/10.3390/ijms19123785
Ren W, Qiao Z, Wang H, Zhu L, Zhang L (2003) Flavonoids: promising anticancer agents. Med Res Rev 23:519–534. https://doi.org/10.1002/med.10033
Rigobello MP, Scutari G, Boscolo R, Bindoli A (2002) Induction of mitochondrial permeability transition by auranofin, a gold(I)-phosphine derivative. Br J Pharmacol 136:1162–1168. https://doi.org/10.1038/sj.bjp.0704823
Supuran CT, Maresca A, Greg F, Remko M (2013) Three new aromatic sulfonamide inhibitors of carbonic anhydrases I, II, IV and XII. 28:289–293. https://doi.org/10.3109/14756366.2011.649269
Sze JH, Raninga PV, Nakamura K, Casey M, Khanna KK, Berners-Price SJ, di Trapani G, Tonissen KF (2020) Anticancer activity of a gold(I) phosphine thioredoxin reductase inhibitor in multiple myeloma. Redox Biol 28:101310. https://doi.org/10.1016/j.redox.2019.101310
Taşer P, Çİftcİ, M (2012) Purifi cation and characterization of glutathione reductase from turkey liver 36:546–553. https://doi.org/10.3906/vet-1103-5
Taslimi P, Caglayan C, Gulcin İ (2017) The impact of some natural phenolic compounds on carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase, and α-glycosidase enzymes: an antidiabetic, anticholinergic, and antiepileptic study. J Biochem Mol Toxicol 31:1–7. https://doi.org/10.1002/jbt.21995
Temel Y, Bayindir S (2019) The synthesis of thiosemicarbazone-based aza-ylides as inhibitors of rat erythrocyte glucose 6-phosphate dehydrogenase enzyme. J Inst Sci Technol 9:1503–1512. https://doi.org/10.21597/jist.518012
Temel Y, Kocyigit UM (2017a) Purification of glucose-6-phosphate dehydrogenase from rat (Rattus norvegicus) erythrocytes and inhibition effects of some metal ions on enzyme activity. J Biochem Mol Toxicol 31. https://doi.org/10.1002/jbt.21927
Temel Y, Kocyigit UM (2017b) Purification of glucose-6-phosphate dehydrogenase from rat (Rattus norvegicus) erythrocytes and inhibition effects of some metal ions on enzyme activity. https://doi.org/10.1002/jbt.21927
Temel Y, Taysi MŞ (2018) The effect of mercury chloride and boric acid on rat erythrocyte enzymes. Biol Trace Elem Res 191:177–182. https://doi.org/10.1007/s12011-018-1601-x
Temel Y, Kufrevioǧlu ÖI, Çiftci M (2017a) Investigation of the effects of purification and characterization of turkey (Meleagris gallopavo) liver mitochondrial thioredoxin reductase enzyme and some metal ions on enzyme activity. Turkish J Chem 41:48–60. https://doi.org/10.3906/kim-1603-135
Temel Y, Bozkuş T, Karagözoğlu Y, Çİftcİ, M (2017b) Glutatyon Redüktaz ( GR ) Enziminin Japon Bıldırcın ( Coturnix coturnix japanica ) Eritrositlerinden Saflaştırılması ve Karakterizasyonu Purification and characterization of glutathion reductase enzyme from Japanese Quail (Coturnix coturnix japanica) Er. 7:143–150
Temel Y, Bengü AŞ, Akkoyun HT, Akkoyun M, Ciftci M (2017c) Effect of astaxanthin and aluminum chloride on erythrocyte G6PD and 6PGD enzyme activities in vivo and on erythrocyte G6PD in vitro in rats. J Biochem Mol Toxicol 31. https://doi.org/10.1002/jbt.21954
Temel Y, Ayna A, Hamdi Shafeeq I, Ciftci M (2018) In vitro effects of some antibiotics on glucose-6-phosphate dehydrogenase from rat (Rattus norvegicus ) erythrocyte . Drug Chem Toxicol 0:1–5. https://doi.org/10.1080/01480545.2018.1481083, 43
Topal F, Nar M, Gocer H, Kalin P, Kocyigit UM, Gülçin İ, Alwasel SH (2016) Antioxidant activity of taxifolin: an activity-structure relationship. J Enzyme Inhib Med Chem 31:674–683. https://doi.org/10.3109/14756366.2015.1057723
Türkeş C, Demir Y, Beydemir Ş (2019) Anti-diabetic properties of calcium channel blockers: inhibition effects on aldose reductase enzyme activity. Appl Biochem Biotechnol 189:318–329. https://doi.org/10.1007/s12010-019-03009-x
Urig S, Becker K (2006) On the potential of thioredoxin reductase inhibitors for cancer therapy. Semin Cancer Biol 16:452–465. https://doi.org/10.1016/j.semcancer.2006.09.004
Winterbourn CC (2019) Regulation of intracellular glutathione. Redox Biol 22:101086. https://doi.org/10.1016/j.redox.2018.101086
Zhang J, Li X, Han X, Liu R, Fang J (2017) Targeting the thioredoxin system for cancer therapy. Trends Pharmacol Sci 38:794–808. https://doi.org/10.1016/j.tips.2017.06.001
Zhang D, Liu Y, Luo Z, Chen Y, Xu A, Liang Y, Wu B, Tong X, Liu X, Shen H, Liu L, Wei Y, Zhou H, Liu Y, Zhou F (2020) The novel thioredoxin reductase inhibitor A-Z2 triggers intrinsic apoptosis and shows efficacy in the treatment of acute myeloid leukemia. Free Radic Biol Med 146:275–286. https://doi.org/10.1016/j.freeradbiomed.2019.11.013
Zheng W, Feng Q, Liu J, Guo Y, Gao L, Li R, Xu M, Yan G, Yin Z, Zhang S, Liu S, Shan C (2017) Inhibition of 6-phosphogluconate dehydrogenase reverses cisplatin resistance in ovarian and. Lung Cancer 8:1–11. https://doi.org/10.3389/fphar.2017.00421
Zhou J, Xia L, Zhang Y (2019) Naringin inhibits thyroid cancer cell proliferation and induces cell apoptosis through repressing PI3K/AKT pathway. Pathol Res Pract 215:152707. https://doi.org/10.1016/j.prp.2019.152707
Zhu S, He L, Zhang F, Li M, Jiao S, Li Y, Chen M, Zhao XE, Wang H (2016) Fluorimetric evaluation of glutathione reductase activity and its inhibitors using carbon quantum dots. Talanta 161:769–774. https://doi.org/10.1016/j.talanta.2016.09.048
Acknowledgements
We are grateful to Bingol University, Turkey.
Funding
This study was supported by the Scientific Research Projects Coordination Unit of Bingol University (Project number BAP-SSHMYO.2016.00.001).
Author information
Authors and Affiliations
Contributions
YT and CC conceived and designed the research. BMA and YT conducted the biochemical analyses. YT, CC, FMK, and MC analyzed the data. YT and CC wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Chrysin regulated abnormal increases and decreases in CYP-induced metabolic enzyme activities.
• Naringin regulated abnormal increases and decreases in CYP-induced metabolic enzyme activities.
• G6PD enzyme purified from rat erythrocyte using 2′,5-ADP Sepharose 4B affinity gel.
• Chrysin increased the G6PD enzyme activity in vitro.
• Naringin inhibited the G6PD enzyme activity noncompetitively.
Rights and permissions
About this article
Cite this article
Temel, Y., Çağlayan, C., Ahmed, B.M. et al. The effects of chrysin and naringin on cyclophosphamide-induced erythrocyte damage in rats: biochemical evaluation of some enzyme activities in vivo and in vitro. Naunyn-Schmiedeberg's Arch Pharmacol 394, 645–654 (2021). https://doi.org/10.1007/s00210-020-01987-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01987-y