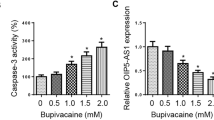
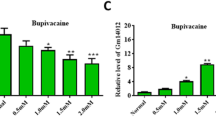
Abstract
This study aims to explore the regulatory mechanisms of dexmedetomidine in parthanatos. MTT assay was applied to reveal cell viability; JC-1 staining assay was utilized to reveal mitochondrial membrane potential. Reactive oxygen species (ROS) probe, DCFH-DA, was used to detect intracellular ROS production. Luciferase activity assay was applied to measure the binding between miR-7-5p and PARP1. We first identified that bupivacaine inhibited the viability and induced the parthanatos of human neuroblastoma SH-SY5Y cells. In addition, dexmedetomidine, a potent α2-adrenoceptor agonist, reversed the regulatory effect of bupivacaine on parthanatos of SH-SY5Y. More importantly, dexmedetomidine counteracted bupivacaine-induced changes of mitochondrial membrane potential and ROS production in SH-SY5Y cells. Hyper-activation of PARP1 plays a vital role in parthanatos. Further exploration of our study identified that bupivacaine triggered overexpression of PARP1 in SH-SY5Y cells. Bioinformatics analysis revealed that miR-7-5p targeted the 3′ untranslated region (3′ UTR) of PARP1 to inhibit PARP1 expression. In addition, dexmedetomidine recovered the suppressive effects of bupivacaine on miR-7-5p expression. Dexmedetomidine suppressed bupivacaine-induced parthanatos in SH-SY5Y cells via the miR-7-5p/PARP1 axis, which may shed a new insight into parthanatos-dependent neuronal injury.






Similar content being viewed by others
Change history
01 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00210-021-02080-8
References
Akhiani AA, Werlenius O, Aurelius J, Movitz C, Martner A, Hellstrand K, Thoren FB (2014) Role of the ERK pathway for oxidant-induced parthanatos in human lymphocytes. PLoS One 9:e89646. https://doi.org/10.1371/journal.pone.0089646
Bacsik CJ, Swift JQ, Hargreaves KM (1995) Toxic systemic reactions of bupivacaine and etidocaine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 79:18–23. https://doi.org/10.1016/s1079-2104(05)80067-8
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. https://doi.org/10.1016/s0092-8674(04)00045-5
Byram SC, Byram SW, Miller NM, Fargo KN (2017) Bupivacaine increases the rate of motoneuron death following peripheral nerve injury. Restor Neurol Neurosci 35:129–135. https://doi.org/10.3233/rnn-160692
David KK, Andrabi SA, Dawson TM, Dawson VL (2009) Parthanatos, a messenger of death. Front Biosci (Landmark Ed) 14:1116–1128. https://doi.org/10.2741/3297
Donnan GA, Fisher M, Macleod M, Davis SM (2008) Stroke. Lancet (London, England) 371:1612–1623. https://doi.org/10.1016/s0140-6736(08)60694-7
Fatokun AA, Dawson VL, Dawson TM (2014) Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol 171:2000–2016. https://doi.org/10.1111/bph.12416
Ge X et al (2018) Hypoxia-mediated mitochondria apoptosis inhibition induces temozolomide treatment resistance through miR-26a/Bad/Bax axis. Cell Death Dis 9:1128. https://doi.org/10.1038/s41419-018-1176-7
Gu J, Chen J, Xia P, Tao G, Zhao H, Ma D (2011) Dexmedetomidine attenuates remote lung injury induced by renal ischemia-reperfusion in mice. Acta Anaesthesiol Scand 55:1272–1278. https://doi.org/10.1111/j.1399-6576.2011.02526.x
Gu N et al (2017) Anti-apoptotic and angiogenic effects of intelectin-1 in rat cerebral ischemia. Brain Res Bull 130:27–35. https://doi.org/10.1016/j.brainresbull.2016.12.006
Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B (2006) Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesthesia 53:646–652. https://doi.org/10.1007/bf03021622
Hu X-b et al (2019) Dexmedetomidine promotes SH-SY5Y cell resistance against impairment of Iron overload by inhibiting NF-κB pathways. 44:959–967. https://doi.org/10.1007/s11064-019-02731-6
Huang YF, Pryor ME, Mather LE, Veering BT (1998) Cardiovascular and central nervous system effects of intravenous levobupivacaine and bupivacaine in sheep. Anesth Analg 86:797–804. https://doi.org/10.1097/00000539-199804000-00023
Jin W, Xu W, Chen J, Zhang X, Shi L, Ren C (2016) Remote limb preconditioning protects against ischemia-induced neuronal death through ameliorating neuronal oxidative DNA damage and parthanatos. J Neurol Sci 366:8–17. https://doi.org/10.1016/j.jns.2016.04.038
Jing N, Huang T, Guo H, Yang J, Li M, Chen Z, Zhang Y (2018) LncRNA CASC15 promotes colon cancer cell proliferation and metastasis by regulating the miR4310/LGR5/Wnt/betacatenin signaling pathway molecular medicine reports. 18:2269–2276. https://doi.org/10.3892/mmr.2018.9191
Lai J, Yang H, Zhu Y, Ruan M, Huang Y, Zhang Q (2019) MiR-7-5p-mediated downregulation of PARP1 impacts DNA homologous recombination repair and resistance to doxorubicin in small cell lung cancer. 19:602. https://doi.org/10.1186/s12885-019-5798-7
Lam SW, Alexander E (2008) Dexmedetomidine use in critical care. AACN Adv Crit Care 19:113–120. https://doi.org/10.1097/01.Aacn.0000318109.06073.35
Lee Y, Kang HC, Lee BD, Lee YI, Kim YP, Shin JH (2014) Poly (ADP-ribose) in the pathogenesis of Parkinson's disease. BMB Rep 47:424–432. https://doi.org/10.5483/bmbrep.2014.47.8.119
Lehner C et al (2013) Bupivacaine induces short-term alterations and impairment in rat tendons. Am J Sports Med 41:1411–1418. https://doi.org/10.1177/0363546513485406
Li L et al (2013) Neuroprotective effect of ginkgolide B on bupivacaine-induced apoptosis in SH-SY5Y cells:159864. https://doi.org/10.1155/2013/159864
Li W, Yang Y, Cheng X, Liu M, Zhang S, Wang Y, GJAaijopcd D (2020) Baicalein attenuates caspase-independent cells death via inhibiting PARP-1 activation and AIF nuclear translocation in cerebral ischemia/reperfusion rats. 25:354–369. https://doi.org/10.1007/s10495-020-01600-w
Liu K et al (2017) Expression levels of atherosclerosis-associated miR-143 and miR-145 in the plasma of patients with hyperhomocysteinaemia. BMC Cardiovasc Disord 17:163. https://doi.org/10.1186/s12872-017-0596-0
Lu P, Kamboj A, Gibson SB, Anderson CM (2014) Poly(ADP-ribose) polymerase-1 causes mitochondrial damage and neuron death mediated by Bnip3. J Neurosci 34:15975–15987. https://doi.org/10.1523/jneurosci.2499-14.2014
Ma D et al (2016) Deoxypodophyllotoxin triggers parthanatos in glioma cells via induction of excessive ROS. Cancer Lett 371:194–204. https://doi.org/10.1016/j.canlet.2015.11.044
Morrison SG, Dominguez JJ, Frascarolo P, Reiz S (2000) A comparison of the electrocardiographic cardiotoxic effects of racemic bupivacaine, levobupivacaine, and ropivacaine in anesthetized swine. Anesth Analg 90:1308–1314. https://doi.org/10.1097/00000539-200006000-00009
Rao PD, Sankrityayan H, Srivastava A, Kulkarni YA, Mulay SR, Gaikwad AB (2020) 'PARP'ing fibrosis: repurposing poly (ADP ribose) polymerase (PARP) inhibitors. Drug Discov Today. https://doi.org/10.1016/j.drudis.2020.04.019
Sha J, Feng X, Chen Y, Zhang H, Li B, Hu X, Fan H (2019) Dexmedetomidine improves acute stress-induced liver injury in rats by regulating MKP-1, inhibiting NF-κB pathway and cell apoptosis. 234:14068–14078. https://doi.org/10.1002/jcp.28096
Shin YJ, Kim JH, Seo JM, Lee SM, Hyon JY, Yu YS, Wee WR (2009) Protective effect of clusterin on oxidative stress-induced cell death of human corneal endothelial cells. Mol Vis 15:2789–2795
Si Y, Bao H, Han L, Shi H, Zhang Y, Xu L, Liu C, Wang J, Yang X, Vohra A, Ma D (2013) Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation. J Transl Med 11:141. https://doi.org/10.1186/1479-5876-11-141
Singh A, Gupta A, Datta PK, Pandey M (2018) Intrathecal levobupivacaine versus bupivacaine for inguinal hernia surgery: a randomized controlled trial. Korean J Anesthesiol 71:220–225. https://doi.org/10.4097/kja.d.18.27191
Sun Y-B et al (2019) Dexmedetomidine inhibits astrocyte pyroptosis and subsequently protects the brain in in vitro and in vivo models of sepsis. 10:167. https://doi.org/10.1038/s41419-019-1416-5
Taylor S, Berkelman T, Yadav G, Hammond MJM (2013) A defined methodology for reliable quantification of Western blot data. 55:217–226. https://doi.org/10.1007/s12033-013-9672-6
Wang Y, Dawson VL, Dawson TM (2009) Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos. Exp Neurol 218:193–202. https://doi.org/10.1016/j.expneurol.2009.03.020
Wang HF et al (2018) Endoplasmic reticulum stress regulates oxygen-glucose deprivation-induced parthanatos in human SH-SY5Y cells via improvement of intracellular ROS. CNS Neurosci Therapeut 24:29–38. https://doi.org/10.1111/cns.12771
Wang T et al (2019) Allopregnanolone modulates GABAAR-dependent CaMKIIdelta3 and BDNF to protect SH-SY5Y cells against 6-OHDA-induced damage. Front Cell Neurosci 13:569. https://doi.org/10.3389/fncel.2019.00569
Wen X, Xu S, Liu H, Zhang Q, Liang H, Yang C, Wang H (2013) Neurotoxicity induced by bupivacaine via T-type calcium channels in SH-SY5Y cells. PLoS ONE 8:e62942. https://doi.org/10.1371/journal.pone.0062942
Whittington RA et al (2015) Dexmedetomidine increases tau phosphorylation under normothermic conditions in vivo and in vitro. 36:2414–2428. https://doi.org/10.1016/j.neurobiolaging.2015.05.002
Wu Q et al (2016) miR-489 inhibits silica-induced pulmonary fibrosis by targeting MyD88 and Smad3 and is negatively regulated by lncRNA CHRF. Sci Rep 6:30921. https://doi.org/10.1038/srep30921
Xiang H, Hu B, Li Z, Li J (2014) Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation 37:1763–1770. https://doi.org/10.1007/s10753-014-9906-1
Zhang H, Lin J, Hu T, Ren Z, Wang W, He Q (2019) Effect of miR-132 on bupivacaine-induced neurotoxicity in human neuroblastoma cell line. J Pharmacol Sci 139:186–192. https://doi.org/10.1016/j.jphs.2019.01.014
Zhang N, Wang D, Yang X, Hou D (2020) Long noncoding RNA small nucleolar RNA host gene 1 contributes to sevoflurane-induced neurotoxicity through negatively modulating microRNA-181b. Neuroreport 31:416–424. https://doi.org/10.1097/wnr.0000000000001430
Zheng T, Lu-Ying L, Li L, Xu S (2016) Parthanatos is involved in bupivacaine induced injury in SH-SY5Y cells. 9:20941–20951. https://www.researchgate.net/publication/311790559_Parthanatos_is_involved_in_bupivacaine_induced_injury_in_SH-SY5Y_cells
Zheng T, Zheng CY, Zheng XC, Zhao RG, Chen YQ (2017) Effect of parthanatos on ropivacaine-induced damage in SH-SY5Y cells clinical and experimental. Pharmacol Physiol 44:586–594. https://doi.org/10.1111/1440-1681.12730
Zheng J, Chen J, Wu G, Wu C, Wang R, Wang W (2018) Inhibiting EZH2 rescued bupivacaine-induced neuronal apoptosis in spinal cord dorsal root ganglia in mice. J Anesth 32:524–530. https://doi.org/10.1007/s00540-018-2506-8
Acknowledgments
We appreciate the support of Fujian Medical University, Fujian Provincial Hospital and Fujian Provincial Emergency Center.
Funding
This work was supported by the
1. The Medical Education Branch of the Chinese Medical Association, the Medical Education Committee of the Chinese Higher Education Society (2016B-LC033)
2. the Natural Science Foundation of Fujian Province (2018J01246)
3. the Medical Innovation Project of Fujian province (2018-CX-2)
4. high-level hospital foster grants from Fujian Provincial Hospital, Fujian Province, China (2019HSJJ23)
5.high-level hospital foster grants from Fujian Provincial Hospital, Fujian Province, China (2019HSJJ21)
6. the Training Project for Talents of Fujian Provincial Health Commission (2019-ZQN-1)
7.Provincial special subsidy funds for health care of Fujian Provincial Department of Finance (No. 2020-467)
8. Fujian Science and Technology Innovation Joint Fundation (Major Program)(2019Y9028)
Author information
Authors and Affiliations
Contributions
Study concepts and design: Ting Zheng and Xiao-chun Zheng
Data acquisition and analysis: Ting Zheng, Chunying Zheng, and Fei Gao
Experimental studies and statistical analysis: Ting Zheng, Chunying Zheng, Fei Gao, and Fengyi Huang
Manuscript preparation and editing: Ting Zheng, Chunying Zheng, Fei Gao, Fengyi Huang, and Bin Hu
Manuscript review: Ting Zheng, Chunying Zheng, Fei Gao, Fengyi Huang, Bin Hu, and Xiaochun Zheng
All data were generated in-house and did not use a paper mill.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure 1
(A) Cell viability by treatment of Bupivacaine for different time was revealed. N = 5. (B) RT-qPCR revealed expression of MIR7-3HG by Bupivacaine and (or) Dexmedetomidine treatment. #p < 0.05 compared with DMSO group; Фp < 0.05 compared with Bupivacaine group or Dexmedetomidine group. N = 5. (C) RT-qPCR revealed expression of EZH2 by Bupivacaine and (or) Dexmedetomidine treatment. #p < 0.05 compared with DMSO group; Фp < 0.05 compared with Bupivacaine group or Dexmedetomidine group. N = 5. (D) RT-qPCR revealed expression of EZH2 by transfection of EZH2#1/2 in Bupivacaine (10 μM) treated SH-SY5Y cells. #p < 0.05 compared with sh-NC group. (E-F) RT-qPCR revealed the influence of EZH2#1/2 on expression of MIR7-3HG and miR-7-5p in Bupivacaine (10 μM) treated SH-SY5Y cells. #p < 0.05 compared with sh-NC group. (G) Luciferase reporter assay revealed activity of MIR7-3HG promoter under the influence of EZH2#1/2 in Bupivacaine (10 μM) treated cells. #p < 0.05 compared with sh-NC group. (H) ChIP assay revealed relative enrichment of MIR7-3HG promoter precipitated by IgG, EZH2 and H3K27me3. #p < 0.05 compared with IgG group. (PNG 459 kb)
ESM 1
(PDF 298 kb)
Rights and permissions
About this article
Cite this article
Zheng, T., Zheng, C., Gao, F. et al. Dexmedetomidine suppresses bupivacaine-induced parthanatos in human SH-SY5Y cells via the miR-7-5p/PARP1 axis-mediated ROS. Naunyn-Schmiedeberg's Arch Pharmacol 394, 783–796 (2021). https://doi.org/10.1007/s00210-020-01971-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01971-6