Abstract
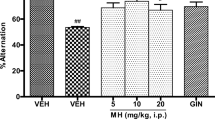
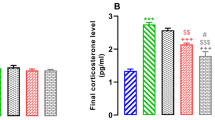
Unpredictable chronic mild stress (UCMS) has been shown to cause memory loss via increased oxidative stress and deregulation of monoaminergic and cholinergic neurotransmissions. Although the benefits of methyl jasmonate (MJ), a well-known anti-stress plant hormone against chronic stress-induced psychopathologies, have been earlier reported, its effects on antioxidant defense molecules, monoaminergic transmitters, and nuclear factor erythroid 2-related factor 2 (Nrf2) immunopositive cells have not been extensively studied. The present study was designed to examine its effect on memory functions, antioxidant biomarkers, monoaminergic transmitters, and Nrf2 immunopositive cell expression in rats exposed to UCMS. Rats received an intraperitoneal injection of MJ (10, 25, and 50 mg/kg) 30 min before exposure to UCMS daily for 28 days. Memory function was assessed on day 29 using a modified elevated plus maze and novel object recognition tests. The antioxidant biomarkers, level of monoamines (serotonin, noradrenaline, and dopamine), and Nrf2 immunopositive cell expression were determined in the rat brain tissues. The activity of cholinesterase and monoamine oxidase enzymes was also determined. MJ attenuated memory deficits and elevated the brain levels of monoamines in UCMS rats. UCMS-induced increase of brain cholinesterase and monoamine oxidase activities was inhibited by MJ. Also, MJ attenuated UCMS-induced decrease in antioxidant enzymes (CAT, GPx, GST, and SOD) and thiol contents in the brains of rats. UCMS-induced increase in NO level and Nrf2 immunopositive cell expression in the rat’s brain was attenuated by MJ. Taken together, these findings suggest that increasing antioxidant defense molecules and monoaminergic/cholinergic neurotransmitters and decreasing the Nrf2 immunopositive cell expressions may contribute to the memory-promoting effects of MJ in rats exposed to UCMS.









Similar content being viewed by others
References
Adebesin A, Adeoluwa OA, Eduviere AT, Umukoro S (2017) Methyl jasmonate attenuated lipopolysaccharide-induced depressive-like behaviour in mice. J Psych Res 94:29–35
Adeniyi PA, Ishola AO, Laoye BJ, Olatunji BP, Bankole OO, Shallie PD, Ogundele OM (2016) Neural and behavioral changes in male preadolescent mice after prolonged nicotine-MDMA treatment. Metabolic Brain Disease 30:15
Aluko OM, Umukoro S, Annafi O, Adewole FA, Omorogbe O (2015) Effects of methyl jasmonate on acute stress responses in mice subjected to forced swim and anoxic tests. Sci Pharm 83:635–644
Baker GB, Matveychuk D, MacKenzie EM, Dursun SM, Mousseau DD (2012) Monoamine oxidase inhibitors and neuroprotective mechanisms. Bull Clin Psychopharmacol 22:293–296
Bowles D (1990) Defense-related proteins in higher plants. Annu Rev Biochem 59:873–907
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I (2015) Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology (Berlin) 232:931–942
Cesari IM, Carvalho E, Rodrigues MF, Mendonça MS, Amôedo ND, Rumjanek FD (2014) Methyl jasmonate: putative mechanisms of action on cancer cells cycle, metabolism, and apoptosis. Int J Cell Bio 2014:572097
Claiborne A (1985) Handbook of methods for oxygen free radical research, CRC press, Boca Raton, R.a. Greenwald (Ed.), 283-284
Dinkova-Kostova AT, Kostov RV, Kazantsev AG (2018) The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J 285:3576–3590
Eduviere AT, Umukoro S, Aderibigbe AO, Ajayi AM, Adewole FA (2015) Methyl jasmonate enhances memory performance through inhibition of oxidative stress and acetylcholinesterase activity in mice. Life Sci 132:20–26
El-Awdan SA, Abdel Jaleel GA, Saleh DO (2015) Alleviation of haloperidol-induced oxidative stress in rats: effects of sucrose vs grape seed extract. Bull Fac Pharm Cairo Univ 53(1):29–35
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ennaceur A (2010) One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav Brain Res 215(2):244–254
Epp JR, Chow C, Galea LA (2013) Hippocampus-dependent learning influences hippocampal neurogenesis. Front Neurosci 7:57
Erukainure OL, Ijomone OM, Oyebode OA, Chukwuma CI, Aschner M, Islam S (2019) Hyperglycemia-induced oxidative brain injury: therapeutic effects of Cola nitida-infusion against redox imbalance, cerebellar neuronal insults, and upregulated Nrf2expression in type 2 diabetic rats. Food&Chem Tox
Fingrut O, Reischer D, Rotem R, Goldin N, Altboum I, Zan-Bar I, Flescher E (2005) Jasmonates induce nonapoptotic death in high-resistance mutant p53-expressing B-lymphoma cells. Brit J Pharm 146(6):800–808
Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64:97–112
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferase the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Henckens MJ, Hermans AG, Pu EJ, Joels ZM, Fernandez G (2009) Stressed memories: how acute stress affects memory formation in humans. J Neurosci 29(32):10111–10119
Hlinak Z, Krejci I (2002) MK-801 induced amnesia for the elevated plus-maze in mice. Behav Brain Res 131:221–225
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex J Med 54(4):287–293
Joshi G, Biederman J, Wozniak J, Goldin RL, Crowley D, Furtak S, Lukas SE, Gönenç A (2013) Magnetic resonance spectroscopy study of the glutamatergic system in adolescent males with high-functioning autistic disorder: a pilot study at 4T. Eur Arch Psychiatry Clin Neurosci 263:379–384
Kalia M (2005) Neurobiological basis of depression: an update. Metab 54(Suppl 1):24–27
Kaludercic N, Carpi A, Menabo R, Di-Lisa F, Paolocci N (2011) Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta (BBA) Mol Cell Res 1813:1323–1332
Kerksick C, Willoughby D (2005) The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc of Sports Nutri 2(2):38–44
Kim MJ, Kim SS, Park KJ et al (2016) Methyl jasmonate inhibits lipopolysaccharide-induced inflammatory cytokine production via mitogenactivated protein kinase and nuclear factor-κB pathways in RAW 2647 cells. Die Pharmazie 71(9):540–543
Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC (2001) Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 56(9):1143–53
Leger MQ, Bouet A, Haelewyn V, Boulouard B, Schumann-Bard MP, Freret T (2013) Object recognition test in mice. Nat Protoc 8(12):2531–2537
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795
Loizzo M, Tundis R, Menichini F, Menichini F (2008) Natural Products and their Derivatives as Cholinesterase Inhibitors in the Treatment of Neurodegenerative Disorders An Update. Curr Med Chem 15(12):1209–1228
Lovinger DM (2010) Neurotransmitter roles in synaptic modulation, plasticity, and learning in the dorsal striatum. Neuropharmacology 58:951–961
Lueptow LM (2017) Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J Vis Exp (126)
Ma Q (2013) Role of Nrf2 in Oxidative Stress and Toxicity. Annual Review of Pharmacology and Toxicology 53:401–26
Marisco PC, Carvalho FB, Rosa MM, Girardi BA, Gutierres JM, Jaques JA, Salla APS, Pimentel VC, Schetinger MRC, Leal DBR, Mello CF, Rubin MA (2013) Piracetam prevents scopolamine-induced memory impairment and decrease of NTPDase, 5′-nucleotidase and adenosine deaminase activities. Neurochem Res 38:1704–1714
McEwen BS, Nasca C, Gray JD (2016) Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacol Rev 41:3–23
Mejia-Carmona GE, Gosselink KL, Perez-Ishiwara G, Martinez-Martinez A (2015) Oxidant/antioxidant effects of chronic exposure to predator odor in prefrontal cortex, amygdala, and hypothalamus. Mol Cell Biochem 406:121–129
Mercier S, Canini F, Buguet A, Cespuglio R, Martin S (2003) Bourdon L corrigendum to “Behavioural changes after an acute stress: stressor and test types influences”. Behav Brain Res 139:167–175
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5(1):62–71
Mizuki D, Matsumoto K, Tanaka K, Thile X, Fujiwara H, Ishikawa T, Higuchi Y (2014) Antidepressant-like effect of Butea superba in mice exposed to chronic mild stress and its possible mechanism of action. J Ethnopharmacol 156:16–25
Mohandas J, Marshall JJD, Horvath GG, Tiller JS, Tiller DJ (1984) Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res 44:5086–5091
Monleau M, De Méo M, Frelon S, Paquet F, Donnadieu-Claraz M, Duménil G, Chazel V (2006) Distribution and Genotoxic effects after successive exposure to different uranium oxide particles inhaled by rats. Inhal Toxicol 18(11):885–894
Munhoz CD, García-Bueno B, JLM M, Lepsch LB, Scavone C, Leza JC (2008) Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Braz J Med Biol Res 41(12):1037–1046
Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH (2007) Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacol 192:61–70
Nutt DJ (2008) Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry 69:4–7
Nwanna EE, Oyeleye SI, Ogunsuyi OB, Oboh G, Boligon AA, de Athay ML (2016) vitro neuroprotective properties of some commonly consumed green leafy vegetables in Southern Nigeria. NFS Journal 2:19–24
Pani L, Porcella A, Gessa GL (2000) The role of stress in the pathophysiology of the dopaminergic system. Mol Psychiatry 5(1):14–21
Panossian A, Wikman G (2011) Evidence based efficacy of adaptogens in fatigue and molecular mechansims related to their to stress protective activity. Curr Clin Pharmacol 4(3):198–219
Pawar VS, Hugar S (2012) A current status of adaptogens: natural remedy to stress. Asian Pacific J Trop Dis 2:S480–S490
Pitsikas N (2015) The role of nitric oxide in the object recognition memory. Behav Brain Res 285:200–207
Rand MD (2010) Drosophotoxicolgy: the growing potential for Drosophila in neurotoxicology. Neurotoxicology and Teratology 6:74–83
Rinwa P, Kumar A (2014) Modulation of nitrergic signalling pathway by American ginseng attenuate chronic unpredictable stress-induced cognitive impairment, neuroinflammation, and biochemical alterations. Naunyn Schmiedeberg's Arch Pharmacol 387:129–141
Rotem R, Heyfets A, Fingrut O, Blickstein D, Shaklai M, Flescher E (2005) Jasmonates: Novel Anticancer Agents Acting Directly and Selectively on Human Cancer Cell Mitochondria. Cancer Research 65(5):1984–1993
Sayre LM, Perry G, Smith MA (2008) Oxidative stress and neurotoxicity. Chem Res Toxicol 21:172–188
Schetinger MR, Morsch VM, Bonan C, Wyse AT (2007) NTPDase and 50 -nucleotidase activities in physiological and disease conditions: new perspectives for human health. BioFactors 31:77–98
Schurr A, Livne A (1976) Differential inhibition of mitochondrial monoamine oxidase from brain by hashish components. Biochem Pharmacol 25(10):1201–1203
Souza LC, Antunes MS, Filho CB, Del Fabbro L, de Gomes MG, Goes AT (2015) Flavonoid chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol Biochem Behav 134:22–30
Thomas T (2000) Monoamine oxidase-B inhibitors in the treatment of Alzheimer's disease. Neurobiol Aging 21:343–348
Tian T, Wang Z, Zhang J (2017) Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. OxidMed Cell Longev 3:1–18
Tuominen V, Tolonen T, Isola J (2012) Immunomembrane: a publicly available web application for digital image analysis of HER2 immunohistochemistry. Histopathol 60:758–767
Umukoro S, Eduviere AT, Aladeokin AC (2012) Anti-aggressive activity of methyl jasmonate and the probable mechanism of its action in mice. Pharmacology Biochemistry and Behavior 101(2):271–277
Umukoro S, Aluko OM, Eduviere AT, Owoeye O (2016) Evaluation of adaptogenic-like property of methyl jasmonate in mice exposed to unpredictable chronic mild stress. Brain Res Bull 121:105–114
Willner P (2017) Validity, reliability, and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacol 134:319–329
Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton CGMB (2001) Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal 3(2):203–213
Yu-Xia L, Li J, Wang Z-Z, Xia CY, Chen N-H (2018) Glucocorticoid receptor activation induces decrease of hippocampal astrocyte number in rats. Psychopharmacology 235:2529–2540
Zhao S, Edwards J, Carroll J, Wiedholz L, Millstein RA, Jiang C, Murphy DL, Lanthorn TH, Holmes A (2006) Insertion mutation at the C-terminus of the serotonin transporter disrupts brain serotonin function and emotion-related behaviors in mice. Neuroscience 140:321–334
Zhu L-J, Liu M-Y, Li H, Liu X, Chen C, Han Z, Zhou Q-G (2014) The Different Roles of Glucocorticoids in the Hippocampus and Hypothalamus in Chronic Stress-Induced HPA Axis Hyperactivity. PLoS One 9(5):e97689
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We acknowledge our technical staff for their support during the experiments.
Author contribution statement
The authors declare that all data were generated in-house and that no paper mill was used.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We report that there are no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 17 kb)
Rights and permissions
About this article
Cite this article
Aluko, O.M., Umukoro, S. Methyl jasmonate reverses chronic stress-induced memory dysfunctions through modulation of monoaminergic neurotransmission, antioxidant defense system, and Nrf2 expressions. Naunyn-Schmiedeberg's Arch Pharmacol 393, 2339–2353 (2020). https://doi.org/10.1007/s00210-020-01939-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-01939-6