Abstract
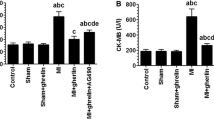
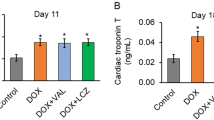
This study investigated if JAK/STAT signaling pathway mediates doxorubicin (DOX)-induced cell death and fibrosis in left ventricles (LVs) of rats and examined if acylated ghrelin affords protection by modulating this pathway. Male rats (120 ± 5 g) were divided into 6 groups (10 rats each) as follows: control; control + AG (10 ng/kg, s.c.); DOX (an accumulative dose 15 mg/kg, i.p.); DOX + AG, DOX + AG + AG490, a JAK2 inhibitor (5 mg/kg, i.p.); and DOX + AG + [D-Lys3]-GHRP-6; an AG receptor antagonist (3.75 mg/kg, i.p.). All treatments were carried out for 35 days. In rats’ LVs, DOX significantly impaired the systolic and diastolic functions, enhanced levels of ROS and MDA, reduced levels of GSH and Bcl-2, and increased mRNA and protein levels of collagen I/III and TGF-β and cleaved caspase-3. In addition, although DOX did not affect JAK1 or JAK2 activity, it significantly increased protein levels of IL-6, decreased STAT3 and p-STAT3 (Tyr701&Ser727), and increased STAT1 and p-STAT1 (Tyr701&Ser727) levels, with a concomitant decrease in ERK1/2 activity and an increase in P38 activity. However, without affecting IL-6 and JAK1/2, AG reversed all of the observed alterations with a significant increase in the levels and activities of JAK2. Similar effects of AG were also seen in control rats. Interestingly, all the beneficial effects afforded by AG were abolished by AG490 and AG + [D-Lys3]-GHRP-6. In conclusion, DOX-induced cardiac toxicity involves stimulation of IL-6, P38, and STAT1 signaling levels whereas the protective effect afforded by AG involves the activation of ERK1/2 and JAK2/STAT3 and inhibition of STAT1.









Similar content being viewed by others
References
Accornero F, van Berlo JH, Correll RN, Elrod JW, Sargent MA, York A, Rabinowitz JE, Leask A, Molkentin JD (2015) Genetic analysis of connective tissue growth factor as an effector of transforming growth factor β signaling and cardiac remodeling. Mol Cell Biol 35:2154–2164
Al Massadi O, Tschöp MH, Tong J (2011) Ghrelin acylation and metabolic control. Peptides 32:2301–2308
Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M (2004) Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci U S A 101:6975–6980
Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, Malan D, Baj G, Granata R, Broglio F (2002) Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol 159:1029–1037
Barry SP, Townsend PA, Latchman DS, Stephanou A (2007) Role of the JAK–STAT pathway in myocardial injury. Trends Mol Med 13:82–89
Berra E, Diaz-Meco MT, Moscat J (1998) The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem 273:10792–10797
Bisi G, Podio V, Valetto MR, Broglio F, Bertuccio G, Aimaretti G, Pelosi E, Del Rio G, Muccioli G, Ong H, Boghen MF, Deghenghi R, Ghigo E (1999) Cardiac effects of hexarelin in hypopituitary adults. Eur J Pharmacol 381:31–38
Booz GW, Day JN, Baker KM (2002) Interplay between the cardiac renin angiotensin system and JAK-STAT signaling: role in cardiac hypertrophy, ischemia/reperfusion dysfunction, and heart failure. J Mol Cell Cardiol 34:1443–1453
Bressenot A, Marchal S, Bezdetnaya L, Garrier J, Guillemin F, Plénat F (2009) Assessment of apoptosis by immunohistochemistry to active caspase-3, active caspase-7, or cleaved PARP in monolayer cells and spheroid and subcutaneous xenografts of human carcinoma. J Histochem Cytochem 57:289–300
Chang L, Ren Y, Liu X, Li WG, Yang J, Geng B, Weintraub NL, Tang C (2004a) Protective effects of ghrelin on ischemia/reperfusion injury in the isolated rat heart. J Cardiovasc Pharmacol 43:165–170
Chang L, Zhao J, Li GZ, Geng B, Pan CS, Qi YF, Tang CS (2004b) Ghrelin protects myocardium from isoproterenol-induced injury in rats. Acta Pharmacol Sin 25:1131–1137
Costa-Pereira AP, Tininini S, Strobl B, Alonzi T, Schlaak JF, Is' harc H, Gesualdo I, Newman SJ, Kerr IM, Poli V (2002) Mutational switch of an IL-6 response to an interferon-γ-like response. Proc Natl Acad Sci U S A 99:8043–8047
Dai B, Cui M, Zhu M, Su W-L, Qiu M-C, Zhang H (2013) STAT1/3 and ERK1/2 synergistically regulate cardiac fibrosis induced by high glucose. Cell Physiol Biochem 32:960–971
Deepak C, Saroj K, Surendra Kumar S, Man Kumar T, Soumya B, Chandra Bhushan J (2016) Effect of doxorubicin on histomorphology of liver of Wistar albino rats. JPP 4:186–190
Desai VG, Herman EH, Moland CL, Branham WS, Lewis SM, Davis KJ, George NI, Lee T, Kerr S, Fuscoe JC (2013) Development of doxorubicin-induced chronic cardiotoxicity in the B6C3F1 mouse model. Toxicol Appl Pharmacol 266:109–121
Eid RA, Alkhateeb MA, Eleawa S, Al-Hashem FH, Al-Shraim M, El-Kott AF, Zaki MSA, Dallak MA, Aldera H (2018) Cardioprotective effect of ghrelin against myocardial infarction-induced left ventricular injury via inhibition of SOCS3 and activation of JAK2/STAT3 signaling. Basic Res Cardiol 113:13
Fischer P, Hilfiker-Kleiner D (2008) Role of gp130-mediated signalling pathways in the heart and its impact on potential therapeutic aspects. Br J Pharmacol 153:S414–S427
Frascarelli S, Ghelardoni S, Ronca-Testoni S, Zucchi R (2003) Effect of ghrelin and synthetic growth hormone secretagogues in normal and ischemic rat heart. Basic Res Cardiol 98:401–405
Granata R, Settanni F, Biancone L, Trovato L, Nano R, Bertuzzi F, Destefanis S, Annunziata M, Martinetti M, Catapano F (2007) Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3′,5′-cyclic adenosine monophosphate/protein kinase a, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-kinase/Akt signaling. Endocrinology 148:512–529
Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J-i (2005) G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med 11:305–311
Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E (2004) Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res 95:187–195
Hiura Y, Takiguchi S, Yamamoto K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Miyata H, Fujiwara Y, Mori M, Kangawa K, Doki Y (2012) Effects of ghrelin administration during chemotherapy with advanced esophageal cancer patients: a prospective, randomized, placebo-controlled phase 2 study. Cancer 118:4785–4794
Hong YM, Lee H, Cho M-S, Kim KC (2017) Apoptosis and remodeling in adriamycin-induced cardiomyopathy rat model. Korean J Pediatr 60:365–372
Huang CX, Yuan MJ, Huang H, Wu G, Liu Y, Yu SB, Li HT, Wang T (2009) Ghrelin inhibits post-infarct myocardial remodeling and improves cardiac function through anti-inflammation effect. Peptides 30:2286–2291
Işeri SO, Sener G, Saglam B, Ercan F, Gedik N, Yeğen BC (2008) Ghrelin alleviates biliary obstruction-induced chronic hepatic injury in rats. Regul Pept 146(1–3):73–79
Jacoby JJ, Kalinowski A, Liu M-G, Zhang SS-M, Gao Q, Chai G-X, Ji L, Iwamoto Y, Li E, Schneider M (2003) Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc Natl Acad Sci U S A 100:12929–12934
Johnson TA, Singla DK (2018) PTEN inhibitor VO-OHpic attenuates inflammatory M1 macrophages and cardiac remodeling in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 315:H1236
Kihara M, Kaiya H, Win ZP, Kitajima Y, Nishikawa M (2016) Protective effect of dietary ghrelin-containing salmon stomach extract on mortality and cardiotoxicity in doxorubicin-induced mouse model of heart failure. J Food Sci 81:H2858–H2865
Kojima M, Hosoda H, Matsuo H, Kangawa K (2001) Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab 12:118–122
Kui L, Weiwei Z, Ling L, Daikun H, Guoming Z, Linuo Z, Renming H (2009) Ghrelin inhibits apoptosis induced by high glucose and sodium palmitate in adult rat cardiomyocytes through the PI3K-Akt signaling pathway. Regul Pept 155:62–69
Kumar D, Kirshenbaum LA, Li T, Danelisen I, Singal PK (2001) Apoptosis in adriamycin cardiomyopathy and its modulation by probucol. Antioxid Redox Signal 3:135–146
Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Yamada S, Okabe M, Kishimoto T, Yamauchi-Takihara K (2000) Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc Natl Acad Sci U S A 97:315–319
L’Ecuyer T, Sanjeev S, Thomas R, Novak R, Das L, Campbell W, Heide RV (2006) DNA damage is an early event in doxorubicin-induced cardiac myocyte death. Am J Physiol Heart Circ Physiol 291:H1273–H1280
Lee J, Hong F, Kwon S, Kim SS, Kim DO, Kang HS, Lee SJ, Ha J, Kim SS (2002) Activation of p38 MAPK induces cell cycle arrest via inhibition of Raf/ERK pathway during muscle differentiation. Biochem Biophys Res Commun 298:765–771
Li SP, Junttila MR, Han J, Kahari VM, Westermarck J (2003) p38 mitogen-activated protein kinase pathway suppresses cell survival by inducing dephosphorylation of mitogen-activated protein/extracellular signal-regulated kinase kinase1,2. Cancer Res 63:3473–3477
Li Y, Hai J, Li L, Chen X, Peng H, Cao M, Zhang Q (2013) Administration of ghrelin improves inflammation, oxidative stress, and apoptosis during and after non-alcoholic fatty liver disease development. Endocrine 43:376–386
Liu M, Li Y, Liang B, Li Z, Jiang Z, Chu C, Yang J (2018) Hydrogen sulfide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT signaling pathway. Int J Mol Med 41:1867–1876
Lou H, Danelisen I, Singal PK (2005) Involvement of mitogen-activated protein kinases in adriamycin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol 57:H1925
Lu Z, Xu S (2006) ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 58:621–631
Mantawy EM, El-Bakly WM, Esmat A, Badr AM, El-Demerdash E (2014) Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol 728:107–118
Masson PJ (1929) Some histological methods: trichrome stainings and their preliminary technique. J Tech Methods 12:75
Mousseaux D, Le Gallic L, Ryan J, Oiry C, Gagne D, Fehrentz J-A, Galleyrand J-C, Martinez J (2009) Regulation of ERK1/2 activity by ghrelin-activated growth hormone secretagogue receptor 1A involves a PLC/PKCɛ pathway human GHSR-1a-mediated ERK1/2 activation. Br J Pharmacol 148:350–365
Nagaya N, Kojima M, Uematsu M, Yamagishi M, Hosoda H, Oya H, Hayashi Y, Kangawa K (2001a) Hemodynamic and hormonal effects of human ghrelin in healthy volunteers. Am J Physiol Regul Integr Comp Physiol 280:R1483–R1487
Nagaya N, Uematsu M, Kojima M, Ikeda Y, Yoshihara F, Shimizu W, Hosoda H, Hirota Y, Ishida H, Mori H, Kangawa K (2001b) Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation 104:1430–1435
Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K (2004) Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 110:3674–3679
Nanzer A, Khalaf S, Mozid A, Fowkes R, Patel M, Burrin J, Grossman A, Korbonits M (2004) Ghrelin exerts a proliferative effect on a rat pituitary somatotroph cell line via the mitogen-activated protein kinase pathway. Eur J Endocrinol 151:233–240
Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, Osugi T, Izumi M, Oshima Y, Nakaoka Y (2001) Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation 104:979–981
Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL (2012) Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol 52:1213–1225
Pei XM, Yung BY, Yip SP, Ying M, Benzie IF, Siu PM (2013) Desacyl ghrelin prevents doxorubicin-induced myocardial fibrosis and apoptosis via the GHSR-independent pathway. Am J Physiol Endocrinol Metab 306:E311–E323
Pei XM, Yung BY, Yip SP, Chan LW, Wong CS, Ying M, Siu PM (2015) Protective effects of desacyl ghrelin on diabetic cardiomyopathy. Acta Diabetol 52:293–306
Pipicz M, Demján V, Sárközy M, Csont T (2018) Effects of cardiovascular risk factors on cardiac STAT3. Int J Mol Sci 19:3572
Podewski EK, Hilfiker-Kleiner D, Hilfiker A, Morawietz H, Lichtenberg A, Wollert KC, Drexler H (2003) Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation 107:798–802
Poizat C, Puri PL, Bai Y, Kedes L (2005) Phosphorylation-dependent degradation of p300 by doxorubicin-activated p38 mitogen-activated protein kinase in cardiac cells. Mol Cell Biol 25:2673–2687
Riad A, Bien S, Westermann D, Becher PM, Loya K, Landmesser U, Kroemer HK, Schultheiss HP, Tschöpe C (2009) Pretreatment with statin attenuates the cardiotoxicity of doxorubicin in mice. Cancer Res 69:695–699
Rossi F, Castelli A, Bianco MJ, Bertone C, Brama M, Santiemma V (2009) Ghrelin inhibits contraction and proliferation of human aortic smooth muscle cells by cAMP/PKA pathway activation. Atherosclerosis 203:97–104
Sauter KAD, Wood LJ, Wong J, Iordanov M, Magun BE (2011) Doxorubicin and daunorubicin induce processing and release of interleukin-1 beta through activation of the NLRP3 inflammasome. Cancer Biol Ther 11:1008–1016
Scarabelli TM, Mariotto S, Abdel-Azeim S, Shoji K, Darra E, Stephanou A, Chen-Scarabelli C, Marechal JD, Knight R, Ciampa A (2009) Targeting STAT1 by myricetin and delphinidin provides efficient protection of the heart from ischemia/reperfusion-induced injury. FEBS Lett 583:531–541
Shizukuda Y, Matoba S, Mian OY, Nguyen T, Hwang PM (2005) Targeted disruption of p53 attenuates doxorubicin-induced cardiac toxicity in mice. Mol Cell Biochem 273:25–32
Stephanou A, Brar BK, Scarabelli TM, Jonassen AK, Yellon DM, Marber MS, Knight RA, Latchman DS (2000) Ischemia-induced STAT-1 expression and activation play a critical role in cardiomyocyte apoptosis. J Biol Chem 275:10002–10008
Wallace KB (2003) Doxorubicin-induced cardiac mitochondrionopathy. Pharmacol Toxicol 93:105–115
Wang X, Shaw S, Amiri F, Eaton DC, Marrero MB (2002) Inhibition of the Jak/STAT signaling pathway prevents the high glucose-induced increase in TGF-β and fibronectin synthesis in mesangial cells. Diabetes 51:3505–3509
Wang X, Wang X-L, Chen H-L, Wu D, Chen J-X, Wang X-X, Li R-L, He J-H, Mo L, Cen X, Wei Y-Q, Jiang W (2014) Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem Pharmacol 88:334–350
Wu Z, Li W, Sun Y, Fu K, Cheng S (2017) Eleutheroside E inhibits doxorubicin-induced inflammation and apoptosis in rat cardiomyocytes by modulating activation of NF-κB pathway. Trop J Pharm Res 16:515–523
Xiang Y, Li Q, Li M, Wang W, Cui C, Zhang J (2011) Ghrelin inhibits AGEs induced apoptosis in human endothelial cells involving ERK1/2 and PI3K/Akt pathways. Cell Biochem Funct 29:149–155
Xu Z, Wu W, Zhang X, Liu G (2007) Endogenous ghrelin increases in adriamycin-induced heart failure rats. J Endocrinol Investig 30:117–125
Xu Z, Lin S, Wu W, Tan H, Wang Z, Cheng C, Lu L, Zhang X (2008) Ghrelin prevents doxorubicin-induced cardiotoxicity through TNF-alpha/NF-κB pathways and mitochondrial protective mechanisms. Toxicology 247:133–138
Yang C, Liu Z, Liu K, Yang P (2014) Mechanisms of ghrelin antiheart failure: inhibition of Ang II induced cardiomyocyte apoptosis by down-regulating AT1R expression. PLoS One 9:e85785
Zhang G, Yin X, Qi Y, Pendyala L, Chen J, Hou D, Tang C (2010) Ghrelin and cardiovascular diseases. Curr Cardiol Rev 6:62–70
Acknowledgments
The authors would like to thank the animal facility staff at the King Khalid University (KKU), Abha, KSA, for their help in taking care of the animals, treatment, and blood tissue collection. They would like also to thank Mr. Mahmoud Alkhateeb from the College of Medicine at King Saud University of Health Sciences and the technical staff members of the Physiology and Biochemistry in the College of Medicine at KKU for their contribution in the recording of the cardiovascular function of the experimental groups and helping in the determination of some biochemical parameters. Furthermore, the authors would like to thank Dr. Reffat Eid, from the Pathology Department at the College of Medicine in KKU for his significant contribution in the histology, immunohistochemistry, and electron microscopy studies. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the research group program under grant number (R.G.P.1 /40/39).
Funding
This study was funded by the Deanship of Scientific Research at King Khalid University, Abha, Saudi Arabia (grant number R.G.P.1/40/39).
Author information
Authors and Affiliations
Contributions
AS conceived and designed the research. AS and AFE conducted the experiments. AS analyzed and graphed the data. AS and AFEK wrote and revised the manuscript. AS and AFE read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards for the use and care of laboratory animals at King Khalid University, Abha, KSA.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shati, A.A., El-kott, A.F. Acylated ghrelin prevents doxorubicin-induced cardiac intrinsic cell death and fibrosis in rats by restoring IL-6/JAK2/STAT3 signaling pathway and inhibition of STAT1. Naunyn-Schmiedeberg's Arch Pharmacol 392, 1151–1168 (2019). https://doi.org/10.1007/s00210-019-01664-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01664-9