Abstract
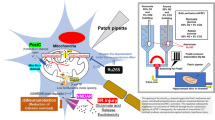
Pinacidil, a nonselective ATP-sensitive K+ (KATP) channel opener, has cardioprotective effects for hypertension, ischemia/reperfusion injury, and arrhythmia. This agent abolishes early afterdepolarizations, delayed afterdepolarizations (DADs), and abnormal automaticity in canine cardiac ventricular myocytes. DADs are well known to be caused by the Na+/Ca2+ exchange current (INCX). In this study, we used the whole-cell patch-clamp technique and Fura-2/AM (Ca2+-indicator) method to investigate the effect of pinacidil on INCX in isolated guinea pig cardiac ventricular myocytes. In the patch-clamp study, pinacidil enhanced INCX in a concentration-dependent manner. The half-maximal effective concentration values were 23.5 and 23.0 μM for the Ca2+ entry (outward) and Ca2+ exit (inward) components of INCX, respectively. The pinacidil-induced INCX increase was blocked by L-NAME, a nitric oxide (NO) synthase inhibitor, by ODQ, a soluble guanylate cyclase inhibitor, and by KT5823, a cyclic guanosine monophosphate (cGMP)-dependent protein kinase (PKG) inhibitor, but not by N-2-mercaptopropyonyl glycine (MPG), a reactive oxygen species (ROS) scavenger. Glibenclamide, a nonselective KATP channel inhibitor, blocked the pinacidil-induced INCX increase, while 5-HD, a selective mitochondria KATP channel inhibitor, did not. In the Fura-2/AM study pinacidil also enhanced intracellular Ca2+ concentration, which was inhibited by L-NAME, ODQ, KT5823, and glibenclamide, but not by MPG and 5-HD. Sildenafil, a phosphodiesterase 5 inhibitor, increased further the pinacidil-induced INCX increase. Sodium nitroprusside, a NO donor, also increased INCX. In conclusion, pinacidil may stimulate cardiac Na+/Ca2+ exchanger (NCX1) by opening plasma membrane KATP channels and activating the NO/cGMP/PKG signaling pathway.







Similar content being viewed by others
References
Ago Y, Kawasaki T, Nashida T, Ota Y, Cong Y, Kitamoto M, Takahashi T, Takuma K, Matsuda T (2011) SEA0400, a specific Na+/Ca2+ exchange inhibitor, prevents dopaminergic neurotoxicity in an MPTP mouse model of Parkinson’s disease. Neuropharmacology 61:1441–1451
Baczkó I, Giles WR, Light PE (2004) Pharmacological activation of plasma-membrane KATP channels reduces reoxygenation-induced Ca2+ overload in cardiac myocytes via modulation of the diastolic membrane potential. Br J Pharmacol 141:1059–1067
Bers DM (2000) Calcium fluxes involved in control of cardiac myocyte contraction. Cir Res 87:275–281
Blaustein MP, Lederer WJ (1999) Sodium/calcium exchange: its physiological implications. Physiol Rev 79:763–854
Carlsson L, Abrahamsson C, Drews L, Duker G (1992) Antiarrhythmic effects of potassium channel openers in rhythm abnormalities related to delayed repolarization. Circulation 85:1491–1500
Chen S-J, Wu C-C, Yang S-N, Lin C-I, Yen M-H (2000) Abnormal activation of K+ channels in aortic smooth muscle of rats with endotoxic shock: electrophysiological and function evidence. Br J Pharmacol 131:213–222
Cuong DV, Kim N, Youm JB, Joo H, Warda M, Lee JW, Park WS, Kim T, Kang S, Kim H, Han J (2006) Nitric oxide-cGMP-protein kinase G signaling pathway induces anoxic preconditioning through activation of ATP-sensitive K+ channels in rat hearts. Am J Physiol Heart Circ Physiol 290:H1808–H1817
Eigel BN, Gursahani H, Hardley RW (2004) ROS are required for rapid reactivation of Na+/Ca2+ exchanger in hypoxic reoxygenated Guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol 286:H955–H963
Fernandes G, Dasai N, Kozlova N, Mojadadi A, Gall M, Drew E, Barratt E, Madamidola OA, Brown SG, Milne AM, Martins da Siva SJ, Whalley KM, Barratt CLR, Jovanovic A (2016) A spontaneous increase in intracellular Ca2+ in metaphase II human oocytes in vitro can be prevented by drugs targeting ATP-sensitive K+ channels. Hum Reprod 31:287–297
Foster MN, Coetzee WA (2016) KATP channel in the cardiovascular system. Physiol Rev 96:177–252
Furukawa K, Ohshima N, Tawada-Iwata Y, Shigekawa M (1991) Cyclic GMP stimulates Na+/Ca2+ exchange in vascular smooth muscle cells in primary culture. J Biol Chem 266:12337–12341
Gendron ME, Thorin E, Perrault LP (2004) Loss of endothelial KATP channel-dependent, NO-mediated dilation of endocardial resistance coronary arteries in pigs with left ventricular hypertrophy. Br J Pharmacol 143:285–291
Goldhaber JI (1996) Free radical enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol 271:H823–H833
Gonzalez DR, Treuer A, Sun Q-A, Stamler JS, Hare JM (2009) S-nitrosylation of cardiac ion channels. J Cardiovasc Pharmacol 54:188–195
Han J, Kim N, Joo H, Kim E, Earm Y (2002a) ATP-sensitive K+ channel activation by nitric oxide and protein kinase G in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol 283:H1545–H1554
Han J, Kim N, Park J, Seog D-H, Joo H, Kim E (2002b) Opening of mitochondrial ATP-sensitive potassium channels evokes oxygen radical generation in rabbit heart slices. J Biochem 131:721–727
Hinata M, Matsuoka I, Iwamoto T, Watanabe Y, Kimura J (2007) Mechanism of Na+/Ca2+ exchanger activation by hydrogen peroxide in Guinea-pig ventricular myocytes. J Pharmacol Sci 103:283–292
Iwamoto T, Nakamura TY, Pan Y, Uehara A, Imanaga I, Shigekawa M (1999) Unique topology of the internal repeats in the cardiac Na+-Ca2+ exchanger. FEBS Lett 446:264–268
Kitao T, Takuma K, Kawasaki T, Inoue Y, Ikehara A, Nashida T, Ago Y, Matsuda T (2010) The Na+/Ca2+ exchanger-mediated Ca2+ influx triggers nitric oxide-induced cytotoxicity in cultured astrocytes. Neurochem Int 57:58–66
Krenz M, Oldenburg O, Wimpee H, Cohen MV, Garlid KD, Critz SD, Downey JM, Benoit JN (2002) Opening of ATP-sensitive potassium channels causes generation of free radicals in vascular smooth muscle cells. Basic Res Cardiol 97:365–373
Lebuffe G, Schumacker PT, Shao Z-U, Anderson T, Iwase H, Vanden Hoek TL (2003) ROS and NO trigger early preconditioning: relationship to mitochondrial KATP channel. Am J Physiol Heart Circ Physiol 284:H299–H308
Liu D, Homan LL, Dillon JS (2004) Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5′-monophosphate-dependent mechanism. Endocrinology 145:5532–5539
Mączewski M, Beręsewicz A (1997) Inhibitors of nitric oxide synthesis and ischemia/reperfusion attenuate coronary vasodilator response to pinacidil in isolated rat heart. J Physiol Pharmacol 48:737–749
Murphy ME, Brayden JE (1995) Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J Physiol 486:47–58
Newgreen DT, Bray KM, McHarg AD, Weston AH, Duty S, Brown BS, Kay PB, Edwards G, Longmore J, Southerton JS (1990) The action of diazoxide and minoxidil sulphate on rat blood vessels: a comparison with cromakalim. Br J Pharmacol 100:605–613
Nicoll DA, Ottolia M, Lu L, Lu Y, Philipson KD (1999) A new topological model of the cardiac sarcolemmal Na+-Ca2+ exchanger. J Biol Chem 274:910–917
Noda Y, Mori A, Packer L (1997) Gliclazide scavenges hydroxyl, superoxide and nitric oxide radicals: an ESR study. Res Commun Mol Pathol Pharmacol 96:115–124
Oldenburg O, Yang XM, Krieg T, Garlid KD, Cohen MV, Grover GJ, Downey JM (2003) P1075 opens mitochondria KATP channels and generates reactive oxygen species resulting in cardioprotection of rabbit hearts. J Mol Cell Cardiol 35:1035–1042
Reppel M, Fleischmann BK, Reuter H, Sasse P, Schunkert H, Hescheler J (2007) Regulation of the Na+/Ca2+ exchanger (NCX) in the murine embryonic heart. Cardiovasc Res 75:99–108
Secondo A, Molinaro P, Pannaccione A, Esposito A, Cantile M, Lippiello P, Sirabella R, Iwamoto T, Di Renzo G, Annunziato L (2011) Nitric oxide stimulates NCX1 and NCX2 but inhibits NCX3 isoform by three distinct molecular determinants. Mol Pharmacol 79:558–568
Southerton JS, Weston AH, Bray KM, Newgreen DT, Taylor SG (1988) The potassium channel opening action of pinacidil: studies using biochemical, ion flux and microelectrode techniques. Naunyn Schmiedeberg Arch Pharmacol 338:310–318
Spinelli W, Sorota S, Siegal M, Hoffman BF (1991) Antiarrhythmic actions of the ATP-regulated K+ current activated by pinacidil. Circ Res 68:1127–1137
Wei J, Watanabe Y, Takeuchi K, Yamashita K, Tashiro M, Kita S, Iwamoto T, Watanabe H, Kimura J (2016) Nicorandil stimulates a Na+/Ca2+ exchanger by activating guanylate cyclase in Guinea pig cardiac myocytes. Pflugers Arch 468:693–703
Wu Y, He J-K, Y S-H, Ma S-Y, Huang W, Wei Y-Y, Kong H, Wang H, Zeng X-N, Xie W-P (2017) Activation of ATP-sensitive potassium channels facilitates the function of human endothelial colony-forming cells via Ca2+/Akt/eNOS pathway. J Cell Mol Med 21:609–620
Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL (1996) Nitric oxide synthase generates nitric oxide and superoxide in arginine-deleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA 93:6770–6774
Xuan Y-T, Tang X-L, Qiu Y, Banerjee S, Takano H, Han H, Bolli R (2000) Biphasic response of cardiac NO synthase isoforms to ischemic preconditioning in conscious rabbits. Am J Physiol Heart Circ Physiol 279:H2360–H2371
Yamashita K, Watanabe Y, Kita S, Iwamoto T, Kimura J (2016) Inhibitory effect of YM-244769, a novel Na+/Ca2+ exchanger inhibitor on Na+/Ca2+ exchange current in Guinea-pig cardiac ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol 389:1205–1214
Zeitz O, Maass AE, Van Nguyen P, Hensmann G, Kogler H, Moller K, Hasenfuss G, Janssen PM (2002) Hydroxyl radical-induced acute diastolic dysfunction is due to calcium overload via reverse-mode Na+/Ca2+ exchange. Circ Res 90:988–995
Zhang B, Zhang Z, Ji H, Shi H, Chen S, Yan D, Jiang X, Shi B (2015) Grape seed proanthocyanidin extract alleviates urethral dysfunction in diabetic rats through modulating the NO-cGMP pathway. Exp Ther Med 15:1053–1061
Zhang DM, Chai Y, Erickson JR, Brown JH, Bers DM, Lin YF (2014) Intracellular signaling mechanism responsible for modulation of sarcolemmal ATP-sensitive potassium channels by nitric oxide in ventricular cardiomyocytes. J Physiol 592(5):971–990
Acknowledgments
We thank Dr. Junko Kimura and Prof. Yuichi Hattori for helpful and critical comments on the manuscript. This study was supported by Grant-in-Aids for Scientific Research (17 K11047, 16 K09428) from the Japan Society for Promotion of Science.
Author information
Authors and Affiliations
Contributions
M.S., Y.M., and Y.W. conceived and designed the experiments. K.I., K.Y., H.P., and M.S. performed the experiments. K.I., K.Y., and Y.W. analyzed the data. K.I. and Y.W. wrote the article. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iguchi, K., Saotome, M., Yamashita, K. et al. Pinacidil, a KATP channel opener, stimulates cardiac Na+/Ca2+ exchanger function through the NO/cGMP/PKG signaling pathway in guinea pig cardiac ventricular myocytes. Naunyn-Schmiedeberg's Arch Pharmacol 392, 949–959 (2019). https://doi.org/10.1007/s00210-019-01642-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-019-01642-1