Abstract
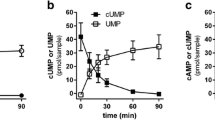
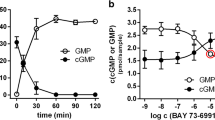
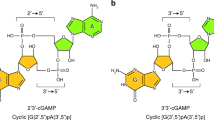
Previous results indicate that the phosphodiesterase PDE3B hydrolyzes cUMP. Also, almost 50 years ago, cUMP-hydrolytic activity was observed in rat adipose tissue. We intended to characterize the enzyme kinetics of PDE3B-mediated cUMP hydrolysis, to determine the PDE3B binding mode of cUMP, and to analyze cUMP hydrolysis in adipocyte preparations. Educts (cNMPs) and products (NMPs) of the PDE reactions as well as intracellular cNMPs were quantitated by HPLC-coupled tandem mass spectrometry. PDE3B expression was determined by qPCR and Western blot. Docking studies were performed with the PDE3B crystal structure PDB ID 1SO2 (complex with a dihydropyridazine inhibitor). PDE3B hydrolyzed cUMP (Km ~ 550 μM, Vmax ~ 76 μmol/min/mg) and cAMP (Km ~ 0.7 μM, Vmax ~ 4.3 μmol/min/mg) in a milrinone (PDE3-selective inhibitor)-sensitive manner (Ki for inhibition of cUMP hydrolysis: 205 nM). cUMP forms one hydrogen bond with PDE3B (uracil 3-NH with side chain oxygen of Q988). Two hydrogen bonds stabilize cAMP binding. cCMP does not interact with PDE3B. Possibly, the cytosine base cannot form hydrogen bonds with PDE3B, and the 4-NH2 group clashes with L987 of the enzyme. Adipocyte differentiation of 3T3-L1 MBX cells increased mRNA of PDE3B, but not of PDE3A. Significant amounts of cUMP were detected in differentiated and undifferentiated 3T3-L1 MBX cells. 3T3-L1 MBX adipocyte lysates and rat epididymal adipose tissue membranes contained milrinone-sensitive cUMP-hydrolytic activity. PDE3B is a low-affinity and high-velocity phosphodiesterase for cUMP. The cUMP-hydrolyzing activity described almost 50 years ago for rat adipose tissue is caused by PDE3, probably by the isoform PDE3B.





Similar content being viewed by others
Abbreviations
- 3T3-L1 :
-
MBX cells
murine pre-adipocyte cell line (subclone of the 3T3-L1 cell line)
- AMP :
-
adenosine 5′-monophosphate
- BSA :
-
bovine serum albumin
- cAMP :
-
adenosine 3′,5′-cyclic monophosphate
- cCMP :
-
cytidine 3′,5′-cyclic monophosphate
- C/EBPα :
-
CCAAT/enhancer-binding protein alpha
- cGMP :
-
guanosine 3′,5′-cyclic monophosphate
- CMP :
-
cytidine 5′-monophosphate
- cNMP :
-
nucleoside 3′,5′-cyclic monophosphate
- cUMP :
-
uridine 3′,5′-cyclic monophosphate
- DMEM :
-
Dulbecco’s modified Eagle medium (cell culture medium)
- DMSO :
-
dimethylsulfoxide
- EDTA :
-
ethylenediaminetetraacetic acid
- ExoY :
-
Exotoxin of Pseudomonas aeruginosa with nucleotidyl cyclase properties
- FABP4 :
-
fatty acid binding protein 4 (also known as adipocyte protein 2 or aP2)
- FCS :
-
fetal calf serum
- GMP :
-
guanosine 5′-monophosphate
- GST :
-
glutathione S-transferase (used as protein tag)
- HCN :
-
hyperpolarization-activated cyclic nucleotide-gated channel
- HPLC-MS/MS :
-
high-performance liquid chromatography-coupled tandem mass spectrometry
- IBMX :
-
3-isobutyl-1-methylxanthine
- K m :
-
Michaelis-Menten constant
- MRP :
-
multidrug resistance-associated protein
- NCS :
-
neonatal calf serum
- NMP :
-
nucleoside 5′-monophosphate
- PBS :
-
phosphate-buffered saline
- PDE :
-
phosphodiesterase
- PKA :
-
protein kinase A
- PKG :
-
protein kinase G
- PPARγ :
-
peroxisome proliferator-activated receptor gamma
- Pref-1 :
-
preadipocyte factor 1
- P/S + G :
-
penicillin/streptomycin + glutamine
- qPC :
-
quantitative real-time PCR
- sAC :
-
soluble adenylyl cyclase
- SD :
-
standard deviation
- SEM :
-
standard error of the mean
- sGC :
-
soluble guanylyl cyclase
- UMP :
-
uridine 5'-monophosphate
- Vmax :
-
maximum velocity of an enzymatic reaction under saturating conditions
References
Ahmad F, Degerman E, Manganiello VC (2012) Cyclic nucleotide phosphodiesterase 3 signaling complexes. Horm Metab Res 44:776–785
Azevedo MF, Faucz FR, Bimpaki E, Horvath A, Levy I, de Alexandre RB, Ahmad F, Manganiello V, Stratakis CA (2014) Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr Rev 35:195–233
Beckert U, Wolter S, Hartwig C, Bähre H, Kaever V, Ladant D, Frank DW, Seifert R (2014) ExoY from Pseudomonas aeruginosa is a nucleotidyl cyclase with preference for cGMP and cUMP formation. Biochem Biophys Res Commun 450:870–874
Bender AT, Beavo JA (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58:488–520
Berrisch S, Ostermeyer J, Kaever V, Kälble S, Hilfiker-Kleiner D, Seifert R, Schneider EH (2017) cUMP hydrolysis by PDE3A. Naunyn Schmiedeberg's Arch Pharmacol 390:269–280
Boswell-Smith V, Spina D, Page CP (2006) Phosphodiesterase inhibitors. Br J Pharmacol 147(Suppl 1):S252–S257
Bähre H, Danker KY, Stasch JP, Kaever V, Seifert R (2014) Nucleotidyl cyclase activity of soluble guanylyl cyclase in intact cells. Biochem Biophys Res Commun 443:1195–1199
Bähre H, Hartwig C, Munder A, Wolter S, Stelzer T, Schirmer B, Beckert U, Frank DW, Tümmler B, Kaever V, Seifert R (2015) cCMP and cUMP occur in vivo. Biochem Biophys Res Commun 460:909–914
Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108
Chung C, Varnerin JP, Morin NR, MacNeil DJ, Singh SB, Patel S, Scapin G, Van der Ploeg LH, Tota MR (2003) The role of tryptophan 1072 in human PDE3B inhibitor binding. Biochem Biophys Res Commun 307:1045–1050
Clark M, Cramer RDI, Van Opdenbosch N (1989) Validation of the general purpose tripos 5.2 force field. J Comput Chem 10:982–1012
Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA (1995) A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc 117:5179–5197
Degerman E, Belfrage P, Manganiello VC (1997) Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3). J Biol Chem 272:6823–6826
Degerman E, Belfrage P, Newman AH, Rice KC, Manganiello VC (1987) Purification of the putative hormone-sensitive cyclic AMP phosphodiesterase from rat adipose tissue using a derivative of cilostamide as a novel affinity ligand. J Biol Chem 262:5797–5807
Deng Y, Wang ZV, Gordillo R, An Y, Zhang C, Liang Q, Yoshino J, Cautivo KM, De Brabander J, Elmquist JK, Horton JD, Hill JA, Klein S, Scherer PE (2017) An adipo-biliary-uridine axis that regulates energy homeostasis. Science 355:eaaf5375. https://doi.org/10.1126/science.aaf5375
Gerhold D, Liu F, Jiang G, Li Z, Xu J, Lu M, Sachs J, Bagchi A, Fridman A, Holder D, Doebber T, Berger J, Elbrecht A, Moller D, Zhang B (2002) Gene expression profile of adipocyte differentiation and its regulation by peroxisome proliferator-activated receptor-gamma agonists. Endocrinology 143:2106–2118
Ghose AK, Viswanadhan VN, Wendoloski JJ (1998) Prediction of hydrophobic (lipophilic) properties of small organic molecules using fragmental methods: an analysis of ALOGP and CLOGP methods. J Phys Chem A 102:3762–3772
Godinho RO, Duarte T, Pacini ES (2015) New perspectives in signaling mediated by receptors coupled to stimulatory G protein: the emerging significance of cAMP efflux and extracellular cAMP-adenosine pathway. Front Pharmacol 6:58
Grant PG, Colman RW (1984) Purification and characterization of a human platelet cyclic nucleotide phosphodiesterase. Biochemistry 23:1801–1807
Harrison SA, Reifsnyder DH, Gallis B, Cadd GG, Beavo JA (1986) Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Mol Pharmacol 29:506–514
Hasan A, Danker KY, Wolter S, Bähre H, Kaever V, Seifert R (2014) Soluble adenylyl cyclase accounts for high basal cCMP and cUMP concentrations in HEK293 and B103 cells. Biochem Biophys Res Commun 448:236–240
Heiden W, Moeckel G, Brickmann J (1993) A new approach to analysis and display of local lipophilicity/hydrophilicity mapped on molecular surfaces. J Comput Aided Mol Des 7:503–514
Hunter RW, Mackintosh C, Hers I (2009) Protein kinase C-mediated phosphorylation and activation of PDE3A regulate cAMP levels in human platelets. J Biol Chem 284:12339–12348
Klotz U, Stock K (1971) Evidence for a cyclic nucleotide-phosphodiesterase with high specificity for cyclic uridine-3′,5′-monophosphate in rat adipose tissue. Naunyn Schmiedeberg's Arch Pharmacol 269:117–120
Laue S, Winterhoff M, Kaever V, van den Heuvel JJ, Russel FG, Seifert R (2014) cCMP is a substrate for MRP5. Naunyn Schmiedeberg's Arch Pharmacol 387:893–895
Lindh R, Ahmad F, Resjö S, James P, Yang JS, Fales HM, Manganiello V, Degerman E (2007) Multisite phosphorylation of adipocyte and hepatocyte phosphodiesterase 3B. Biochim Biophys Acta 1773:584–592
Monzel M, Kuhn M, Bähre H, Seifert R, Schneider EH (2014) PDE7A1 hydrolyzes cCMP. FEBS Lett 588:3469–3474
Norbury W, Herndon DN, Tanksley J, Jeschke MG, Finnerty CC (2016) Infection in burns. Surg Infect 17:250–255
Omori K, Kotera J (2007) Overview of PDEs and their regulation. Circ Res 100:309–327
Oßwald A (2012) Einfluss des mTOR-Signalwegs auf die differentielle microRNA-Expression während der Adipozytendifferenzierung. Doctoral Thesis (MD), Pediatric Clinic and Policlinic, Dr von Hauner's Children's Hospital. Ludwig-Maximilian University, Munich
Reinecke D, Burhenne H, Sandner P, Kaever V, Seifert R (2011) Human cyclic nucleotide phosphodiesterases possess a much broader substrate-specificity than previously appreciated. FEBS Lett 585:3259–3262
Scapin G, Patel SB, Chung C, Varnerin JP, Edmondson SD, Mastracchio A, Parmee ER, Singh SB, Becker JW, Van der Ploeg LH, Tota MR (2004) Crystal structure of human phosphodiesterase 3B: atomic basis for substrate and inhibitor specificity. Biochemistry 43:6091–6100
Seifert R, Schneider EH, Bähre H (2015) From canonical to non-canonical cyclic nucleotides as second messengers: pharmacological implications. Pharmacol Ther 148:154–184
Shakur Y, Holst LS, Landstrom TR, Movsesian M, Degerman E, Manganiello V (2001) Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog Nucleic Acid Res Mol Biol 66:241–277
Smas CM, Sul HS (1993) Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell 73:725–734
Snyder PB, Esselstyn JM, Loughney K, Wolda SL, Florio VA (2005) The role of cyclic nucleotide phosphodiesterases in the regulation of adipocyte lipolysis. J Lipid Res 46:494–503
Taira M, Hockman SC, Calvo JC, Belfrage P, Manganiello VC (1993) Molecular cloning of the rat adipocyte hormone-sensitive cyclic GMP-inhibited cyclic nucleotide phosphodiesterase. J Biol Chem 268:18573–18579
Wang H, Liu Y, Hou J, Zheng M, Robinson H, Ke H (2007a) Structural insight into substrate specificity of phosphodiesterase 10. Proc Natl Acad Sci U S A 104:5782–5787
Wang H, Robinson H, Ke H (2007b) The molecular basis for different recognition of substrates by phosphodiesterase families 4 and 10. J Mol Biol 371:302–307
Wolter S, Golombek M, Seifert R (2011) Differential activation of cAMP- and cGMP-dependent protein kinases by cyclic purine and pyrimidine nucleotides. Biochem Biophys Res Commun 415:563–566
Zebisch K, Voigt V, Wabitsch M, Brandsch M (2012) Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal Biochem 425:88–90
Zhang KY, Card GL, Suzuki Y, Artis DR, Fong D, Gillette S, Hsieh D, Neiman J, West BL, Zhang C, Milburn MV, Kim SH, Schlessinger J, Bollag G (2004) A glutamine switch mechanism for nucleotide selectivity by phosphodiesterases. Mol Cell 15:279–286
Zmuda-Trzebiatowska E, Oknianska A, Manganiello V, Degerman E (2006) Role of PDE3B in insulin-induced glucose uptake, GLUT-4 translocation and lipogenesis in primary rat adipocytes. Cell Signal 18:382–390
Zong X, Krause S, Chen CC, Krüger J, Gruner C, Cao-Ehlker X, Fenske S, Wahl-Schott C, Biel M (2012) Regulation of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel activity by cCMP. J Biol Chem 287:26506–26512
Acknowledgements
We thank Prof. Dr. Harald Genth (Institute of Toxicology, MHH), Dr. Sabine Wolter (Institute of Pharmacology, MHH), and Dr. Thorsten Gnad (Institute of Pharmacology and Toxicology, University of Bonn) for excellent scientific discussions as well as Ms. Annette Garbe (Research Core Unit Metabolomics, MHH) and Ms. Solveig Kälble (Institute of Pharmacology, MHH) for outstanding technical support.
Author information
Authors and Affiliations
Contributions
Enzyme kinetics data: 60% JO, 40% FG
Western blot data: 0% JO, 100% FG
cNMP content of 3T3-L1 cells: 0% JO, 100% FG
qPCR data: 60% JO, 40% FG
Enzyme activity in differentiated 3T3-L1 cells: 0% JO, 100% FG
Enzyme activity in undifferentiated 3T3-L1 cells: 100% JO, 0% FG
Enzyme activity in rat adipose tissue: 100% JO, 0% FG
Molecular modeling: 100% SD
HPLC-MS/MS measurements: 100% VK
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 860 kb)
Rights and permissions
About this article
Cite this article
Ostermeyer, J., Golly, F., Kaever, V. et al. cUMP hydrolysis by PDE3B. Naunyn-Schmiedeberg's Arch Pharmacol 391, 891–905 (2018). https://doi.org/10.1007/s00210-018-1512-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-018-1512-6