Abstract
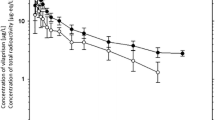
The study was intended to investigate the effect of concomitant administration of antimalarial drug (pyrimethamine or arteether) on pharmacokinetic and post coitus contraceptive efficacy of ormeloxifene in female Sprague-Dawley rats. A serial sampling technique coupled with LC-MS/MS detection was utilized for quantification of ormeloxifene in plasma samples collected from female rats treated with ormeloxifene only and ormeloxifene with pyrimethamine or arteether. Coitus-proven female rats were utilized to investigate the effect of pyrimethamine or arteether coadministration on contraceptive efficacy of ormeloxifene by investigating the presence or absence of implantations and status of corpora lutea on day 10 post coitum. None of the sperm-positive rats treated with ormeloxifene with or without coadministration of pyrimethamine or arteether showed any sign of pregnancy, confirming that concomitant administration of antimalarial drugs (pyrimethamine or arteether) did not affect the pharmacodynamic profile of ormeloxifene. Although there was no sign of pharmacodynamic interaction, the volume of distribution of ormeloxifene increased significantly on cotreatment with pyrimethamine. However, coadministration of arteether did not affect any of the pharmacokinetic parameters of ormeloxifene. The compiled results of preliminary study in female rats support that pyrimethamine or arteether can be prescribed with ormeloxifene.


Similar content being viewed by others
References
Breckenridge AM, Back DJ, Orme M (1979) Interaction between oral contraceptives and other drugs. Pharmacol Ther 7:617–626
Ette EI, Kelman AW, Howie CA, Whiting B (1995) Analysis of animal pharmacokinetic data: performance of the one point per animal design. J Pharmacokinet Biopharm 23:551–566
FDA (2001) Guidance for Industry: Bioanalytical Method Validation http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf. Accessed 1 September 2016
Friedrichs E (2010) On the pill. Nat Med 16:506–508
Ghosh R, Kamboj VP, Singh MM (2001) Interaction with anti-implantation and estrogen antagonistic activities of dl-ormeloxifene, a selective estrogen receptor modulator, by tetracycline in female Sprague-Dawley rats. Contraception 64:261–269
Godfrey KR, Arundel PA, Dong Z, Bryant R (2011) Modelling the double peak phenomenon in pharmacokinetics. Comput Methods Prog Biomed 104:62–69
Kamboj V, Ray S, Dhawan B (1992) Centchroman. Drugs Today 28:227–232
Khurana M, Lal J, Singh MM, Paliwal JK, Kamboj VP, Gupta RC (2002) Evaluation of interaction potential of certain concurrently administered drugs with pharmacological and pharmacokinetic profile of centchroman in rats. Contraception 66:47–56
Kumar V, Lal J, Singh MM, Gupta RC (2006) Effect of concurrently coadministered drugs on the pharmacokinetic/pharmacodynamic profile of centchroman, a nonsteroidal oral contraceptive, in rats. Contraception 74:165–173
Lal J (2010) Clinical pharmacokinetics and interaction of centchroman—a mini review. Contraception 81:275–280
Ray S, Grover PK, Anand N (1971) New synthesis of cis- and trans-3-phenyl-4-[4-(h-pyrolidinoethoxy) phenyl]-7-methoxy chromans. Indian J Chem 9:727–728
Rowland M (1984) Protein binding and drug clearance. Clin Pharmacokinet 9:10–17
Scarsi KK, Darin KM, Nakalema S et al (2016) Unintended pregnancies observed with combined use of the levonorgestrel contraceptive implant and efavirenz-based antiretroviral therapy: a three-arm pharmacokinetic evaluation over 48 weeks. Clin Infect Dis 62:675–682
Sharma A, Jaiswal S, Shukla M, Malik MY, Lal J (2015) Rapid quantitative analysis of ormeloxifene and its active metabolite, 7-desmethyl ormeloxifene, in rat plasma using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 997:7–15
Sharma A, Jaiswal S, Shukla M et al (2016) PK–PD interaction study of angiotensin II antagonist, losartan, with selective estrogen receptor modulator, centchroman. Int J Pharm 1:17–23
Singh M (2001) Centchroman, a selective estrogen receptor modulator, as a contraceptive and for the management of hormone-related clinical disorders. Med Res Rev 21:302–347
Steinkellner A, Chen W, Denison SE (2010) Adherence to oral contraception in women on category X medications. Am J Med 123:929–934 e921
White NJ (1985) Clinical pharmacokinetics of antimalarial drugs. Clin Pharmacokinet 10:187–215
WHO (2015) World Malaria Report 2015. http://who.int/malaria/publications/world-malaria-report-2015/report/en/. Accessed 1 September 2016
Acknowledgments
Author AS is thankful to Indian Council of Medical Research, New Delhi, for financial support (grant number 45/57/2010-PHA/BMS) for the CDRI Communication (9509).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 148 kb).
Rights and permissions
About this article
Cite this article
Sharma, A., Jaiswal, S., Shukla, M. et al. Effect of arteether and pyrimethamine coadministration on the pharmacokinetic and pharmacodynamic profile of ormeloxifene. Naunyn-Schmiedeberg's Arch Pharmacol 390, 971–976 (2017). https://doi.org/10.1007/s00210-017-1401-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-017-1401-4