Abstract
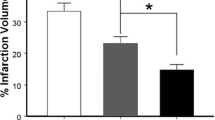
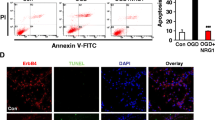
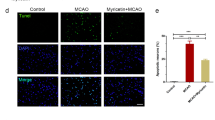
After stroke or traumatic damages, both necrotic and apoptotic neuronal death cause a loss of functions including memory, sensory perception, and motor skills. From the fact that necrosis has a nature to expand, while apoptosis to cease the cell death cascade in the brain, it is considered that the promising target for the rapid treatment for stroke is the necrosis. In this study, I introduce the discovery of prothymosin α (ProTα), which inhibits neuronal necrosis, and propose its potentiality of clinical use for stroke. First of all, it should be noted that ProTα inhibits the neuronal necrosis induced by serum-free starvation or ischemia-reperfusion stress, which causes a rapid internalization of GLUT1/4, leading a decrease in glucose uptake and cellular ATP levels. Underlying mechanisms are determined to be through an activation of Gi/o, phospholipase C and PKCβII. ProTα also causes apoptosis later through a similar mechanism. However, we found that ProTα-induced apoptosis is completely inhibited by the concomitant treatment with neurotrophins, which are up-regulated by ischemic stress in the brain. Of most importance is the finding that the systemic injection of ProTα completely inhibits the brain damages, motor dysfunction and learning memory defect induced by cerebral ischemia-reperfusion stress. As ProTα almost entirely prevents the focal ischemia-induced motor dysfunction 4 h after the start of ischemia, this protein seems to have a promising potentiality for clinical use.







Similar content being viewed by others
Reference
Abramov AY, Scorziello A, Duchen MR (2007) Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci 27:1129–1138
Albrecht J, Hanganu IL, Heck N, Luhmann HJ (2005) Oxygen and glucose deprivation induces major dysfunction in the somatosensory cortex of the newborn rat. Eur J Neurosci 22:2295–2305
Ando A, Yonezawa K, Gout I, Nakata T, Ueda H, Hara K, Kitamura Y, Noda Y, Takenawa T, Hirokawa N et al (1994) A complex of GRB2-dynamin binds to tyrosine-phosphorylated insulin receptor substrate-1 after insulin treatment. EMBO J 13:3033–3038
Ay I, Sugimori H, Finklestein SP (2001) Intravenous basic fibroblast growth factor (bFGF) decreases DNA fragmentation and prevents downregulation of Bcl-2 expression in the ischemic brain following middle cerebral artery occlusion in rats. Brain Res Mol Brain Res 87:71–80
Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C (2003) A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med 9:1180–1186
Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A (2000) Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA 97:10526–10531
Bruer U, Weih MK, Isaev NK, Meisel A, Ruscher K, Bergk A, Trendelenburg G, Wiegand F, Victorov IV, Dirnagl U (1997) Induction of tolerance in rat cortical neurons: hypoxic preconditioning. FEBS Lett 414:117–121
Burkle A (2005) Poly(ADP-ribose). The most elaborate metabolite of NAD+. FEBS J 272:4576–4589
Cheng Y, Deshmukh M, D’Costa A, Demaro JA, Gidday JM, Shah A, Sun Y, Jacquin MF, Johnson EM, Holtzman DM (1998) Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest 101:1992–1999
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219
Danton GH, Dietrich WD (2003) Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol 62:127–136
Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22:391–397
Evstafieva AG, Belov GA, Kalkum M, Chichkova NV, Bogdanov AA, Agol VI, Vartapetian AB (2000) Prothymosin alpha fragmentation in apoptosis. FEBS Lett 467:150–154
Ferri KF, Kroemer G (2001) Organelle-specific initiation of cell death pathways. Nat Cell Biol 3:E255–E263
Fujita R, Ueda H (2003a) Protein kinase C-mediated cell death mode switch induced by high glucose. Cell Death Differ 10:1336–1347
Fujita R, Ueda H (2003b) Protein kinase C-mediated necrosis-apoptosis switch of cortical neurons by conditioned medium factors secreted under the serum-free stress. Cell Death Differ 10:782–790
Fujita R, Ueda H (2007) Prothymosin-alpha1 prevents necrosis and apoptosis following stroke. Cell Death Differ 14:1839–1842
Fujita R, Yoshida A, Mizuno K, Ueda H (2001) Cell density-dependent death mode switch of cultured cortical neurons under serum-free starvation stress. Cell Mol Neurobiol 21:317–324
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D (2002) Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev 54:271–284
Gladstone DJ, Black SE, Hakim AM (2002) Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke 33:2123–2136
Ha HC, Snyder SH (2000) Poly(ADP-ribose) polymerase-1 in the nervous system. Neurobiol Dis 7:225–239
Heales SJ, Bolanos JP, Stewart VC, Brookes PS, Land JM, Clark JB (1999) Nitric oxide, mitochondria and neurological disease. Biochim Biophys Acta 1410:215–228
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736
Jiang X, Kim HE, Shu H, Zhao Y, Zhang H, Kofron J, Donnelly J, Burns D, Ng SC, Rosenberg S, Wang X (2003) Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science 299:223–226
Kaplan DR, Miller FD (2000) Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol 10:381–391
Kintner DB, Luo J, Gerdts J, Ballard AJ, Shull GE, Sun D (2007) Role of Na+-K+-Cl-cotransport and Na+/Ca2 + exchange in mitochondrial dysfunction in astrocytes following in vitro ischemia. Am J Physiol Cell Physiol 292:C1113–C1122
Krause GS, Kumar K, White BC, Aust SD, Wiegenstein JG (1986) Ischemia, resuscitation, and reperfusion: mechanisms of tissue injury and prospects for protection. Am Heart J 111:768–780
Lai AY, Todd KG (2006) Microglia in cerebral ischemia: molecular actions and interactions. Can J Physiol Pharmacol 84:49–59
Li W, Yuan XM, Ivanova S, Tracey KJ, Eaton JW, Brunk UT (2003) 3-Aminopropanal, formed during cerebral ischaemia, is a potent lysosomotropic neurotoxin. Biochem J 371:429–436
Lipton P (1999) Ischemic cell death in brain neurons. Physiol Rev 79:1431–1568
Loh KP, Huang SH, De Silva R, Tan BK, Zhu YZ (2006) Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res 3:327–337
Matsunaga H, Ueda H (2006a) Evidence for serum-deprivation-induced co-release of FGF-1 and S100A13 from astrocytes. Neurochem Int 49:294–303
Matsunaga H, Ueda H (2006b) Voltage-dependent N-type Ca2 + channel activity regulates the interaction between FGF-1 and S100A13 for stress-induced non-vesicular release. Cell Mol Neurobiol 26:237–246
Nakajima K, Kohsaka S (2004) Microglia: neuroprotective and neurotrophic cells in the central nervous system. Curr Drug Targets Cardiovasc Haematol Disord 4:65–84
Patapoutian A, Reichardt LF (2001) Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 11:272–280
Pineiro A, Cordero OJ, Nogueira M (2000) Fifteen years of prothymosin alpha: contradictory past and new horizons. Peptides 21:1433–1446
Slee EA, Zhu H, Chow SC, MacFarlane M, Nicholson DW, Cohen GM (1996) Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem J. 315(Pt 1):21–24
Sofroniew MV, Howe CL, Mobley WC (2001) Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 24:1217–1281
Starkov AA, Chinopoulos C, Fiskum G (2004) Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium 36:257–264
Swanson RA, Ying W, Kauppinen TM (2004) Astrocyte influences on ischemic neuronal death. Curr Mol Med 4:193–205
Taylor CP, Weber ML, Gaughan CL, Lehning EJ, LoPachin RM (1999) Oxygen/glucose deprivation in hippocampal slices: altered intraneuronal elemental composition predicts structural and functional damage. J Neurosci 19:619–629
The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med 333:1581–1587
Tsujimoto Y (2002) Bcl-2 family of proteins: life-or-death switch in mitochondria. Biosci Rep 22:47–58
Tsujimoto Y (2003) Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol 195:158–167
Ueda H, Fujita R (2004) Cell death mode switch from necrosis to apoptosis in brain. Biol Pharm Bull 27:950–955
Ueda M, Fujita R, Koji T, Ueda H (2004) The cognition-enhancer nefiracetam inhibits both necrosis and apoptosis in retinal ischemic models in vitro and in vivo. J Pharmacol Exp Ther 309(1):200–207
Ueda H, Fujita R, Yoshida A, Matsunaga H, Ueda M (2007) Identification of prothymosin-alpha1, the necrosis-apoptosis switch molecule in cortical neuronal cultures. J Cell Biol 176:853–862
Watson RT, Pessin JE (2001) Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog Horm Res 56:175–193
Watson RT, Pessin JE (2006) Bridging the GAP between insulin signaling and GLUT4 translocation. Trends Biochem Sci 31:215–222
White BC, Sullivan JM, DeGracia DJ, O’Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS (2000) Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci 179:1–33
Yamashita T, Sawamoto K, Suzuki S, Suzuki N, Adachi K, Kawase T, Mihara M, Ohsugi Y, Abe K, Okano H (2005) Blockade of interleukin-6 signaling aggravates ischemic cerebral damage in mice: possible involvement of Stat3 activation in the protection of neurons. J Neurochem 94:459–468
Yoshida T, Tomioka I, Nagahara T, Holyst T, Sawada M, Hayes P, Gama V, Okuno M, Chen Y, Abe Y, Kanouchi T, Sasada H, Wang D, Yokota T, Sato E, Matsuyama S (2004) Bax-inhibiting peptide derived from mouse and rat Ku70. Biochem Biophys Res Commun 321:961–966
Zhu H, Fearnhead HO, Cohen GM (1995) An ICE-like protease is a common mediator of apoptosis induced by diverse stimuli in human monocytic THP.1 cells. FEBS Lett 374:303–308
Zorov DB, Juhaszova M, Sollott SJ (2006) Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 1757:509–517
Acknowledgements
I cordially thank Dr Ryousuke Fujita for the great contribution to this study and manuscript. This study was supported by the Grants-in-Aid for Scientific Research (to H.U., B: 13470490 and B: 15390028) on Priority Areas—Research on Pathomechanisms of Brain Disorders (to H.U., 17025031) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and Encouragement of Young Scientists (to R.F., B: 17790066) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueda, H. Prothymosin α plays a key role in cell death mode-switch, a new concept for neuroprotective mechanisms in stroke. Naunyn-Schmied Arch Pharmacol 377, 315–323 (2008). https://doi.org/10.1007/s00210-007-0254-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-007-0254-7