Abstract
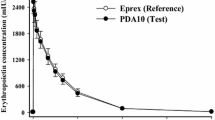
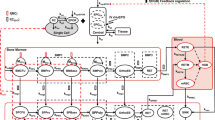
The purpose of this study was to apply the target-mediated drug disposition (TMDD) pharmacokinetic (PK) model to describe binding, internalization, and turnover of erythropoietin receptor (EPOR). This model allows one to determine from free drug (C) PK data not only parameters describing linear disposition of EPO such as the elimination rate constant (k el) and volume of distribution (V c), but also the total receptor concentration (R tot0), drug–receptor complex (RC) internalization rate constant (k int), as well as synthesis and degradation rate constants (k syn and k deg) for the receptor turnover. The previously published data on PK of recombinant EPO (rHuEPO) in humans and the results of EPOR binding studies were used for analysis. The estimated PK parameters were used to simulate time courses of free and bound EPOR after IV administration of clinically relevant rHuEPO doses. The estimates of k el = 0.106 h−1 and V c = 0.032 l/kg are consistent with reported in the literature values of rHuEPO linear disposition parameters. The determined value of R tot0 was 66.35 pM and the half-life for EPOR degradation was 8.8 h. Computer simulations showed a very rapid binding phase in the EPOR time profile followed by a decline to a nadir, and a subsequent return to the baseline. The nadir values decreased with increasing doses and resulted in the maximum values of the bound fractions of the total EPOR in the ranges 33–99%. At the baseline conditions, only 3.1% of EPOR were occupied. The saturation of EPOR was correlated with the time C remained above the K D level. In conclusion, the time courses of serum rHuEPO concentrations contain information about internalization and turnover of EPOR. Kinetics of EPOR can be utilized to determine the relationship between the pharmacologic effect and exposure to rHuEPO.







Similar content being viewed by others
References
Altin JG, White FAJ, Easton CJ (2001) Synthesis of the chelator lipid nitrilotriacetic acid ditetradecylamine (NTA-DTDA) and its use with the IAsys biosensor to study receptor-ligand interactions on model membranes. Biochem Biophys Acta 1513:131–148
Broudy VC, Lin N, Brice M, Nakamoto B, Papayannopoulou T (1991) Erythropoietin receptor characteristics on primary human erythroid cells. Blood 77:2583–2590
Chapel S, Veng-Pedersen P, Hohl RJ, Schmidt RL, McGuire EM, Widness JA (2001) Changes in erythropoietin pharmacokinetics following busulfan-induced bone marrow ablation in sheep: evidence for bone marrow as a major erythropoietin elimination pathway. J Pharmacol Exp Ther 298:820–824
Cheung K, Goon BL, Guilfoyle MC, Wacholtz MC (1998) Pharmacokinetics and pharmacodynamics of recombinant human erythropoietin after single and multiple subcutaneous doses to healthy subjects. Clin Pharmacol Ther 64:412–423
D’Argenio DZ, Schumitzky A (1997) ADAPT II User’s Guide: Pharmacokinetic/Pharmacodynamic System Analysis Software. Biomedical Simulations Resource, Los Angeles
Fisher JW (2003) Erythropoietin: physiology and pharmacology update. Exp Biol Med 228:1–14
Flaharty KK, Caro J, Erslev A, Whalen JJ, Morris EM, Bjornsson TD, Vlasses PH (1990) Pharmacokinetics and erythropoietic response to human recombinant erythropoietin in healthy men. Clin Pharmacol Ther 47:557–564
Glaspy J, Henry D, Patel R, Tchekmedyian S, Applebaum S, Berdeaux D, Lloyd R, Berg R, Austin M, Rossi G, the Darbepoetin Alfa 20010162 Study Group (2005) Effects of chemotherapy on endogenous erythropoietin levels and the pharmacokinetics and erythropoietic response of darbepoetin alfa: a randomized clinical trial of synchronous versus asynchronous dosing of darbepoetin alfa. Eur J Caner 41:1140–1149
Gogu SR, Malter JS, Agrawal KC (1992) Zidovudine induced blockade of the expression and function of the erythropoietin receptor. Biochem Pharmacol 44:1009–1012
Halstenson CE, Macres M, Katz SA, Schnieders JR, Watanabe M, Sobota JT, Abraham PA (1991) Comparative pharmacokinetics and pharmacodynamics of epoetin alfa and epoetin beta. Clin Pharmacol Ther 50:702–712
Jelkmann W (1992) Erythropoietin: structure, control of production, and function. Physiol Rev 72:449–489
Kenakin TP (1987) Pharmacologic analysis of drug-receptor interaction. Raven Press, New York
Kinoshita H, Ohishi N, Kato M, Tokura S, Okazaki A (1992) Pharmacokinetics and distribution of recombinant human erythropoietin in rats. Arzneim-Forsch/Drug Res 42:174–178
Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF (1995) Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell 80:729–38
Krzyzanski W, Jusko WJ, Wacholtz MC, Minton N, Cheung WK (2005) Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after multiple subcutaneous doses in healthy subjects. Eur J Pharm Sci 26:295–306
Lacombe C, Mayeux P (1998) Biology of erythropoietin. Haematologica 83:724–732
Levy G (1994) Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther 56:248–52
Mager DE, Jusko WJ (2001) General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 28:507–532
Mager DE, Wyska E, Jusko WJ (2003) Diversity of mechanism-based pharmacodynamic models. Drug Metab Dispos 31:510–18
Pasqualetti P, Casale R (1996) Circadian rhythm of serum erythropoietin in healthy subjects. Riv Eur Sci Med Farmacol 18:91–93
Pasqualetti P, Collacciani A, Casale R (2000) Circadian rhythm of serum erythropoietin in myelodysplastic syndroms. Riv Eur Sci Med Farmacol 4:111–115
Philo JS, Aoki KH, Arakawa T, Owers Narhi L, Wen J (1996) Dimerization of the extracellular domain of the erythropoietin (EPO) receptor by EPO: one high-affinity and one low-affinity interaction. Biochemistry 35:1681–1691
Piroso E, Erslev AJ, Caro J (1989) Inappropriate increase in erythropoietin titers during chemotherapy. Am J Hematol 32:248–254
Ramakrishnan R, Cheung WK, Farrell F, Joffee L, Jusko WJ (2003) Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after intravenous and subcutaneous dose administration in cynomolgus monkeys. J Pharmacol Exp Ther 306:324–331
Ramakrishnan R, Cheung WK, Wacholtz MC, Winton N, Jusko WJ (2004) Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after single and multiple doses in healthy volunteers. J Clin Pharmacol 44:991–1002
Rowland M, Tozer TN (1995) Clinical pharmacokinetics. Concepts and applications. Williams & Wilkins, Philadelphia
Sawyer ST, Krantz SB, Goldwasser E (1987) Binding and receptor-mediated endocytosis of erythropoietin in Friend virus-infected erythroid cells. J Biol Chem 262:5554–5562
Sugiyama Y, Hanano M (1989) Receptor-mediated transport of peptide hormones and its importance in the overall hormone disposition in the body. Pharm Res 6:192–202
Spivak JL, Cotes PM (1991) The pharmacokinetics and metabolism of erythropoietin. In: Erslev AJ, Adamson JW, Eschbach JW, Winearls CG (eds) Erythropoietin, molecular, cellular, and clinical biology. The John Hopkins University Press, Baltimore, MD
Spivak JL, Barnes DC, Fuchs E, Quinn TC (1989) Serum immunoreactive erythropoietin in HIV-infected patients. J Am Med Assoc 210:3104–3107
Veng-Pedersen P, Chapel S, Al-Huniti NH, Schmidt RL, Sedars EM, Hohl RJ, Widness JA (2003) A differential pharmacokinetic analysis of the erythropoietin receptor population in newborn and adult sheep. J Pharmacol Exp Ther 306:532–537
Walrafen P, Verdier F, Kadri Z, Chretien S, Lacombe C, Mayeux P (2005) Both proteosome and lysosome degrade the activated erythropoietin receptor. Blood 105:600–608
Acknowledgments
This work was supported by grant GM57980 from the National Institutes of Health and funds from the University at Buffalo and Pfizer Strategic Alliance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krzyzanski, W., Wyska, E. Pharmacokinetics and pharmacodynamics of erythropoietin receptor in healthy volunteers. Naunyn-Schmied Arch Pharmacol 377, 637–645 (2008). https://doi.org/10.1007/s00210-007-0225-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-007-0225-z