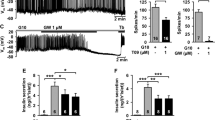
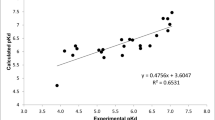
Abstract
The present study investigates the action of zinterol at β3-adrenoceptors. We used mouse primary brown adipocytes and Chinese hamster ovary (CHO-K1) cells expressing the mouse or human β3-adrenoceptor. Zinterol was a full agonist at increasing cyclic AMP levels in primary brown adipocytes (which express β1- and β3-adrenoceptors but not β2-adrenoceptors), and this effect was almost totally abolished in adipocytes derived from β3-adrenoceptor knock-out (KO) mice. Zinterol was also a full agonist at increasing another biological end-point, glucose uptake in brown adipocytes. This effect was reduced in adipocytes derived from β3-adrenoceptor KO mice, with the remaining response sensitive to β1-adrenoceptor antagonism. To determine whether the effect of zinterol on β3-adrenoceptors in primary brown adipocytes can be replicated in a recombinant system, we used CHO-K1 cells expressing the mouse or human β3-adrenoceptor. Zinterol was a full agonist at mouse and human receptors with respect to increasing cyclic AMP levels, with pEC50 values similar to that of the selective β3-adrenoceptor agonist (R, R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]-propyl]1,3-benzodioxole-2,2-dicarboxylate (CL316243) at the mouse receptor. At the human receptor, zinterol was more potent at increasing cyclic AMP levels than CL316243. In cytosensor microphysiometer studies, zinterol was a full agonist for increases in extracellular acidification rates at the mouse and human β3-adrenoceptor. Therefore, we have shown that zinterol is a potent, high-efficacy β3-adrenoceptor agonist at the endogenous mouse β3-adrenoceptor in primary brown adipocytes and at the cloned mouse and human β3-adrenoceptor expressed in CHO-K1 cells. Zinterol is therefore one of few β-adrenoceptor agonists with high potency and efficacy at the human β3-adrenoceptor.





Similar content being viewed by others
Abbreviations
- CHO-K1:
-
Chinese hamster ovary
- CGP12177A:
-
(+)-4-(3-t-butylamino-2-hydroxy-propoxy)benzimidazol-2-one
- CL316243:
-
(R, R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]-propyl]1,3-benzodioxole-2,2-dicarboxylate
- ECAR:
-
extracellular acidification rate
- ICI89406:
-
N-[2-[3-(2-Cyanophenoxy)-2-hydroxypropylamino]ethyl-N´-phenylurea
- KO:
-
knock-out
References
Altschuld RA, Starling RC, Hamlin RL, Billman GE, Hensley J, Castillo L, Fertel RH, Hohl CM, Robitaille PM, Jones LR, Xiao R-P, Lakatta EG (1995) Response of failing canine and human heart cells to β2-adrenergic stimulation. Circulation 92:1612–1618
Arch JR (2002) β3-Adrenoceptor agonists: potential, pitfalls and progress. Eur J Pharmacol 440:99–107
Arch JR, Wilson S (1996) Prospects for β3-adrenoceptor agonists in the treatment of obesity and diabetes. Int J Obes Relat Metab Disord 20:191–199
Arch JR, Ainsworth AT, Cawthorne MA, Piercy V, Sennitt MV, Thody VE, Wilson C, Wilson S (1984) Atypical β-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature 309:163–165
Baker JG (2005a) The selectivity of β-adrenoceptor antagonists at the human β1-, β2- and β3-adrenoceptors. Br J Pharmacol 144:317–322
Baker JG (2005b) Evidence for a secondary state of the human β3-adrenoceptor. Mol Pharmacol 146:2271–2284
Blin N, Camoin L, Maigret B, Strosberg AD (1993) Structural and conformational features determining selective signal transduction in the β3-adrenergic receptor. Mol Pharmacol 44:1094–1104
Bronnikov G, Bengtsson T, Kramarova L, Golozoubova V, Cannon B, Nedergaard J (1999) β1- to β3- switch in control of cyclic adenosine monophosphate during brown adipocyte development explains distinct β-adrenoceptor subtype mediation of proliferation and differentiation. Endocrinology 140:4185–4197
Candelore MR, Deng L, Tota L, Guan XM, Amend A, Liu Y, Newbold R, Cascieri MA, Weber AE (1999) Potent and selective human β3-adrenergic receptor antagonists. J Pharmacol Exp Ther 290:649–655
Carpene C, Galitzky J, Fontana E, Atgie C, Lafontan M, Berlan M (1999) Selective activation of β3-adrenoceptors by octopamine: comparative studies in mammalian fat cells. Naunyn-Schmiedebergs Arch Pharmacol 359:310–321
Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108
Chernogubova E, Cannon B, Bengtsson T (2004) Norepinephrine increases glucose transport in brown adipocytes via β3-adrenoceptors through a cAMP, PKA, and PI3-kinase-dependent pathway stimulating conventional and novel PKCs. Endocrinology 145:269–280
Chernogubova E, Hutchinson DS, Nedergaard J, Bengtsson T (2005) α1- and β1-Adrenoceptor signaling fully compensate for β3-adrenoceptor deficiency in brown adipocyte norepinephrine-stimulated glucose uptake. Endocrinology 146:2271–2284
de Ponti F, Modini C, Gibelli G, Crema F, Frigo G (1999) Atypical β-adrenoceptors mediating relaxation in the human colon: functional evidence for β3- rather than β4-adrenoceptors. Pharmacol Res 39:345–348
Deng C, Paoloni-Giacobino A, Kuehne F, Boss O, Revelli JP, Moinat M, Cawthorne MA, Muzzin P, Giacobino JP (1996) Respective degree of expression of β1-, β2- and β3-adrenoceptors in human brown and white adipose tissues. Br J Pharmacol 118:929–934
Evans BA, Papaioannou M, Hamilton S, Summers RJ (1999) Alternative splicing generates two isoforms of the β3-adrenoceptor which are differentially expressed in mouse tissues. Br J Pharmacol 127:1525–1531
Furchgott RF (1972) The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory. In: Handbook of experimental pharmacology. Springer, Berlin Heidelberg New York pp 283–335
Gauthier C, Langin D, Balligand JL (2000) β3-Adrenoceptors in the cardiovascular system. Trends Pharmacol Sci 21:426–431
Germack R, Adli H, Vassy R, Perret GY (1996) Triiodothyronine and amiodarone effects on β3-adrenoceptor density and lipolytic response to the β3-adrenergic agonist BRL 37344 in rat white adipocytes. Fundam Clin Pharmacol 10:289–297
Heubach JF, Graf EM, Molenaar P, Jager A, Schroder F, Herzig S, Harding SE, Ravens U (2001) Murine ventricular L-type Ca2+ current is enhanced by zinterol via β1-adrenoceptors, and is reduced in TG4 mice overexpressing the human β2-adrenoceptor. Br J Pharmacol 133:73–82
Hirst MA, Pitchford S (1993) Use of a single assay system to assess functional coupling of a variety of receptors. J NIH Res 5:69–73
Hoffmann C, Leitz MR, Oberdorf-Maass S, Lohse MJ, Klotz KN (2004) Comparative pharmacology of human β-adrenergic receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol 369:151–159
Hoffstedt J, Lonnqvist F, Shimizu M, Blaak E, Arner P (1996) Effects of several putative β3-adrenoceptor agonists on lipolysis in human omental adipocytes. Int J Obes Relat Metab Disord 20:428–434
Holloway BR, Howe R, Rao BS, Stribling D, Mayers RM, Briscoe MG, Jackson JM (1991) ICI D7114 a novel selective β-adrenoceptor agonist selectively stimulates brown fat and increases whole-body oxygen consumption. Br J Pharmacol 104:97–104
Hool LC, Harvey RD (1997) Role of β1- and β2-adrenergic receptors in regulation of Cl- and Ca2+ channels in guinea pig ventricular myocytes. Am J Physiol 273:H1669–H1676
Hutchinson DS, Bengtsson T, Evans BA, Summers RJ (2002) Mouse β3a- and β3b-adrenoceptors expressed in Chinese hamster ovary cells display identical pharmacology but utilize distinct signalling pathways. Br J Pharmacol 135:1903–1914
Hutchinson DS, Sato M, Evans BA, Christopoulos A, Summers RJ (2005a) Evidence for pleiotropic signaling at the mouse β3-adrenoceptor revealed by SR59230A [3-(2-ethylphenoxy)-1-[(1,S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propanol oxalate]. J Pharmacol Exp Ther 312:1064–1074
Hutchinson DS, Chernogubova E, Dallner OS, Cannon B, Bengtsson T (2005b) β-Adrenoceptors, but not α-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia 48:2386–2395
Joseph SS, Lynham JA, Colledge WH, Kaumann AJ (2004a) Binding of (–)-[3H]-CGP12177 at two sites in recombinant human β1-adrenoceptors and interaction with β-blockers. Naunyn Schmiedebergs Arch Pharmacol 369:525–532
Joseph SS, Lynham JA, Grace AA, Colledge WH, Kaumann AJ (2004b) Markedly reduced effects of (–)-isoprenaline but not of (–)-CGP12177 and unchanged affinity of β-blockers at Gly389-β1-adrenoceptors compared to Arg389-β1-adrenoceptors. Br J Pharmacol 142:51–56
Juberg EN, Minneman KP, Abel PW (1985) β1- and β2-adrenoceptor binding and functional response in right and left atria of rat heart. Naunyn Schmiedebergs Arch Pharmacol 330:193–202
Kaumann AJ, Sanders L, Lynham JA, Bartel S, Kuschel M, Karczewski P, Krause EG (1996) β2-Adrenoceptor activation by zinterol causes protein phosphorylation, contractile effects and relaxant effects through a cAMP pathway in human atrium. Mol Cell Biochem 163–164:113–123
Konkar AA, Zhu ZX, Granneman JG (2000) Aryloxypropanolamine and catecholamine ligand interactions with the β1-adrenergic receptor: evidence for interaction with distinct conformations of β1-adrenergic receptors. J Pharmacol Exp Ther 294:923–932
Kubota Y, Nakahara T, Yunoki M, Mitani A, Maruko T, Sakamoto K, Ishii K (2002) Inhibitory mechanism of BRL37344 on muscarinic receptor-mediated contractions of the rat urinary bladder smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 366:198–203
Kuznetsov V, Pak E, Robinson RB, Steinberg SF (1995) β2-Adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ Res 76:40–52
Laflamme MA, Becker PL (1998) Do β2-adrenergic receptors modulate Ca2+ in adult rat ventricular myocytes? Am J Physiol 274:H1308–H1314
Leblais V, Pourageaud F, Ivorra MD, Marthan R, Muller B (2005) Comparison of the α-adrenoceptor-mediated effects of β3-adrenoceptor ligands in rat pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol 371:535–539
Levin BE, Sullivan AC (1986) β1-Receptor is the predominant β-adrenoreceptor on rat brown adipose tissue. J Pharmacol Exp Ther 236:681–688
MacLennan SJ, Reynen PH, Martin RS, Eglen RM, Martin GR (2000) Characterization of human recombinant α2A-adrenoceptors expressed in Chinese hamster lung cells using extracellular acidification rate changes. Br J Pharmacol 129:1333–1338
Manara L, Badone D, Baroni M, Boccardi G, Cecchi R, Croci T, Giudice A, Guzzi U, Landi M, Le Fur G (1996) Functional identification of rat atypical β-adrenoceptors by the first β3-selective antagonists, aryloxypropanolaminotetralins. Br J Pharmacol 117:435–442
Mauriege P, De Pergola G, Berlan M, Lafontan M (1988) Human fat cell β-adrenergic receptors: β-agonist-dependent lipolytic responses and characterization of β-adrenergic binding sites on human fat cell membranes with highly selective β1-antagonists. J Lipid Res 29:587–601
Minneman KP, Hedberg A, Molinoff PB (1979) Comparison of β-adrenergic receptor subtypes in mammalian tissues. J Pharmacol Exp Ther 211:502–508
Mohamed-Ali V, Flower L, Sethi J, Hotamisligil G, Gray R, Humphries SE, York DA, Pinkney J (2001) β-Adrenergic regulation of IL-6 release from adipose tissue: in vivo and in vitro studies. J Clin Endocrinol Metab 86:5864–5869
Muzzin P, Revelli JP, Kuhne F, Gocayne JD, McCombie WR, Venter JC, Giacobino JP, Fraser CM (1991) An adipose tissue-specific β-adrenergic receptor. Molecular cloning and down-regulation in obesity. J Biol Chem 266:24053–24058
Nagykaldi Z, Kem D, Lazzara R, Szabo B (1999) Canine ventricular myocyte β2-adrenoceptors are not functionally coupled to L-type calcium current. J Cardiovasc Electrophysiol 10:1240–1251
Nevzorova J, Bengtsson T, Evans BA, Summers RJ (2002) Characterization of the β-adrenoceptor subtype involved in mediation of glucose transport in L6 cells. Br J Pharmacol 137:9–18
Nevzorova J, Evans BA, Bengtsson T, Summers RJ (2006) Multiple signalling pathways involved in β2-adrenoceptor-mediated glucose uptake in rat skeletal muscle cells. Br J Pharmacol 147:446–454
Nisoli E, Tonello C, Carruba MO (1995) Differential relevance of β-adrenoceptor subtypes in modulating the rat brown adipocytes function. Arch Int Pharmacodyn Ther 329:436–453
Nisoli E, Tonello C, Landi M, Carruba MO (1996) Functional studies of the first selective β3-adrenergic receptor antagonist SR 59230A in rat brown adipocytes. Mol Pharmacol 49:7–14
Orcutt AL, Cline TR, Mills SE (1989) Influence of the β2-adrenergic agonist clenbuterol on insulin-stimulated lipogenesis in mouse adipocytes. Domest Anim Endocrinol 6:59–69
Pan SJ, Hancock J, Ding Z, Fogt D, Lee M, Ivy JL (2001) Effects of clenbuterol on insulin resistance in conscious obese Zucker rats. Am J Physiol 280:E554–E561
Pihlavisto M, Scheinin M (1999) Functional assessment of recombinant human α2-adrenoceptor subtypes with cytosensor microphysiometry. Eur J Pharmacol 385:247–253
Popp BD, Hutchinson DS, Evans BA, Summers RJ (2004) Stereoselectivity for interactions of agonists and antagonists at mouse, rat and human β3-adrenoceptors. Eur J Pharmacol 484:323–331
Rehnmark S, Nechad M, Herron D, Cannon B, Nedergaard J (1990) α- and β-adrenergic induction of the expression of the uncoupling protein thermogenin in brown adipocytes differentiated in culture. J Biol Chem 265:16464–16471
Roberts SJ, Papaioannou M, Evans BA, Summers RJ (1999) Characterization of β-adrenoceptor mediated smooth muscle relaxation and the detection of mRNA for β1-, β2- and β3-adrenoceptors in rat ileum. Br J Pharmacol 127:949–961
Rohrer DK, Chruscinski A, Schauble EH, Bernstein D, Kobilka BK (1999) Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptors. J Biol Chem 274:16701–16708
Sillence MN, Moore NG, Pegg GG, Lindsay DB (1993) Ligand binding properties of putative β3-adrenoceptors compared in brown adipose tissue and in skeletal muscle membranes. Br J Pharmacol 109:1157–1163
Skeberdis VA, Jurevicius J, Fischmeister R (1997) β2-Adrenergic activation of L-type Ca2+ current in cardiac myocytes. J Pharmacol Exp Ther 283:452–461
Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, Lowell BB (1995) Targeted disruption of the β3-adrenergic receptor gene. J Biol Chem 270:29483–29492
Weyer C, Gautier JF, Danforth E Jr (1999) Development of β3-adrenoceptor agonists for the treatment of obesity and diabetes-an update. Diabetes Metab 25:11–21
Weyer C, Tataranni PA, Snitker S, Danforth E, Jr., Ravussin E (1998) Increase in insulin action and fat oxidation after treatment with CL 316,243, a highly selective β3-adrenoceptor agonist in humans. Diabetes 47:1555–1561
Xiao RP, Lakatta EG (1993) β1-Adrenoceptor stimulation and β2-adrenoceptor stimulation differ in their effects on contraction, cytosolic Ca2+, and Ca2+ current in single rat ventricular cells. Circ Res 73:286–300
Yanagisawa T, Sato T, Yamada H, Sukegawa J, Nunoki K (2000) Selectivity and potency of agonists for the three subtypes of cloned human β-adrenoceptors expressed in Chinese hamster ovary cells. Tohoku J Exp Med 192:181–193
Zhang ZS, Cheng HJ, Ukai T, Tachibana H, Cheng CP (2001) Enhanced cardiac L-type calcium current response to β2-adrenergic stimulation in heart failure. J Pharmacol Exp Ther 298:188–196
Zhao J, Cannon B, Nedergaard J (1998) Thermogenesis is β3- but not β1-adrenergically mediated in rat brown fat cells, even after cold acclimation. Am J Physiol 275:R2002–R2011
Zhao J, Unelius L, Bengtsson T, Cannon B, Nedergaard J (1994) Coexisting β-adrenoceptor subtypes: significance for thermogenic process in brown fat cells. Am J Physiol 267:C969–C979
Acknowledgements
We thank Martina Helin, Tina Wallin and Maria Uustalu for technical assistance. This study was supported by the Swedish Natural Science Research Council, the Tore Nilsons Stiftelse for Medicinsk Forskening, the Jeanssonska funds and National Health and Medical Research Council of Australia Project Grant 236884. Prof Roger Summers was the 2003 Tage Erlander Gästprofessor of the Swedish Natural Science Research Council and Dr Dana S Hutchinson is a CJ Martin Fellow of the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hutchinson, D.S., Chernogubova, E., Sato, M. et al. Agonist effects of zinterol at the mouse and human β3-adrenoceptor. Naunyn Schmied Arch Pharmacol 373, 158–168 (2006). https://doi.org/10.1007/s00210-006-0056-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-006-0056-3