Abstract
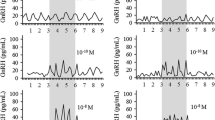
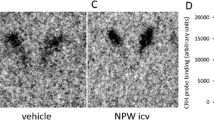
KP-102 (GHRP-2: pralmorelin) is a synthetic growth hormone releasing peptide (GHRP) that powerfully stimulates the release of GH by acting (i.v.) at both hypothalamic and pituitary sites. Intravenous (i.v.) administration of KP-102 also elicits slight but significant release of adrenocorticotropic hormone (ACTH) in both animals and humans, as is seen with other GHRPs. GHRPs are thought to stimulate the hypothalamic-pituitary-adrenal axis by releasing endogenous ACTH secretagogues such as arginine vasopressin (AVP) and/or corticotropin releasing factor (CRF), though neither AVP nor CRF has been shown clearly to be involved significantly in GHRP-evoked ACTH release. In the present study, we investigated the effects of KP-102 on ACTH release in conscious rats under improved experimental conditions that minimized the influence of stress. Administration of KP-102 i.v. increased plasma ACTH significantly, but did not stimulate ACTH release from rat primary pituitary cells. Administration of KP-102 together with either AVP or CRF elicited significantly greater increases in plasma ACTH levels than any of the agonists alone. Notably, the combination of KP-102 and AVP produced a much greater increase in ACTH than KP-102 plus CRF, indicating that KP-102 augments the effect of exogenous CRF only weakly. Conversely, a CRF antagonist markedly inhibited KP-102-induced ACTH release in conscious rats, whereas an AVP antagonist or anti-AVP antiserum did not. Taken together, these findings suggest that KP-102 acts via the hypothalamus to stimulate ACTH release in rats, and that these effects are mediated mainly by the release of CRF.



Similar content being viewed by others
References
Aguilera G, Wynn PC, Harwood JP, Hauger RL, Millan MA, Grewe C, Catt KJ (1986) Receptor-mediated actions of corticotropin-releasing factor in pituitary gland and nervous system. Neuroendocrinology 43:79–88
Aguilera G, Lightman SL, Kiss A (1993) Regulation of the hypothalamic-pituitary-adrenal axis during water deprivation. Endocrinology 132:241–248
Antoni FA (1984) Novel ligand specificity of pituitary vasopressin receptors in the rat. Neuroendocrinology 39:186–188
Arvat E, di Vito L, Maccagno B, Broglio F, Boghen MF, Deghenghi R, Camanni F, Ghigo E (1997) Effects of GHRP-2 and hexarelin, two synthetic GH-releasing peptides, on GH, prolactin, ACTH and cortisol levels in man. Comparison with the effects of GHRH, TRH and hCRF. Peptides 18:885–891
Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M, Katsuura G, Makino S, Fujino MA, Kasuga M (2001) A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology 74:143–147
Bowers CY (1993) GH releasing peptides—structure and kinetics. J Pediatr Endocrinol 6:21–31
Bowers CY (2001) Unnatural growth hormone-releasing peptide begets natural ghrelin. J Clin Endocrinol Metab 86:1464–1469
Broglio F, Benso A, Castiglioni C, Gottero C, Prodam F, Destefanis S, Gauna C, van der Lely AJ, Deghenghi R, Bo M, Arvat E, Ghigo E (2003) The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab 88:1537–1542
Cheng K, Chan WW, Butler B, Wei L, Schoen WR, Wyvratt MJ Jr, Fisher MH, Smith RG (1993) Stimulation of growth hormone release from rat primary pituitary cells by l-692,429, a novel non-peptidyl GH secretagogue. Endocrinology 132:2729–2731
Chu CP, Qiu DL, Kato K, Kunitake T, Watanabe S, Yu NS, Nakazato M, Kannan H (2004) Central stresscopin modulates cardiovascular function through the adrenal medulla in conscious rats. Regul Pept 119:53–59
Clark RG, Robinson IC (1985) Growth hormone responses to multiple injections of a fragment of human growth hormone-releasing factor in conscious male and female rats. J Endocrinol 106:281–289
Deghenghi R, Boutignon F, Luoni M, Grilli R, Guidi M, Locatelli V (1995) Small peptides as potent releasers of growth hormone. J Pediatr Endocrinol Metab 8:311–313
Donaldson CJ, Sutton SW, Perrin MH, Corrigan AZ, Lewis KA, Rivier JE, Vaughan JM, Vale WW (1996) Cloning and characterization of human urocortin. Endocrinology 137:2167–2170
Elias KA, Ingle GS, Burnier JP, Hammonds RG, McDowell RS, Rawson TE, Somers TC, Stanley MS, Cronin MJ (1995) In vitro characterization of four novel classes of growth hormone-releasing peptide. Endocrinology 136:5694–5699
Frieboes RM, Murck H, Maier P, Schier T, Holsboer F, Steiger A (1995) Growth hormone-releasing peptide-6 stimulates sleep, growth hormone, ACTH and cortisol release in normal man. Neuroendocrinology 61:584–589
Gaillard RC, Riondel AM, Ling N, Muller AF (1988) Corticotropin releasing factor activity of CRF 41 in normal man is potentiated by angiotensin II and vasopressin but not by desmopressin. Life Sci 43:1935–1944
Ghigo E, Arvat E, Rizzi G, Goffi S, Grottoli S, Mucci M, Boghen MF, Camanni F (1994) Growth hormone-releasing activity of growth hormone-releasing peptide-6 is maintained after short-term oral pretreatment with the hexapeptide in normal aging. Eur J Endocrinol 131:499–503
Ghigo E, Arvat E, Broglio F, Giordano R, Gianotti L, Muccioli G, Papotti M, Graziani A, Bisi G, Deghenghi R, Camanni F (1999) Endocrine and non-endocrine activities of growth hormone secretagogues in humans. Horm Res 51:9–15
Hartman ML, Farello G, Pezzoli SS, Thorner MO (1992) Oral administration of growth hormone (GH)-releasing peptide stimulates GH secretion in normal men. J Clin Endocrinol Metab 74:1378–1384
Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Van der Ploeg LH (1996) A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273:974–977
Inoue K, Valdez GR, Reyes TM, Reinhardt LE, Tabarin A, Rivier J, Vale WW, Sawchenko PE, Koob GF, Zorrilla EP (2003) Human urocortin II, a selective agonist for the type 2 corticotropin-releasing factor receptor, decreases feeding and drinking in the rat. J Pharmacol Exp Ther 305:385–393
Ishizaki S, Murase T, Sugimula Y, Kakiya S, Yokoi H, Tachikawa K, Arima H, Miura Y, Oiso Y (2002) Role of ghrelin in the regulation of vasopressin release in conscious rats. Endocrinology 143:1589–1593
Jacks T, Hickey G, Judith F, Taylor J, Chen H, Krupa D, Feeney W, Schoen W, Ok D, Fisher M (1994) Effects of acute and repeated intravenous administration of l-692,585, a novel non-peptidyl growth hormone secretagogue, on plasma growth hormone, IGF-1, ACTH, cortisol, prolactin, insulin, and thyroxine levels in beagles. J Endocrinol 143:399–406
Kjaer A (1993) Vasopressin as a neuroendocrine regulator of anterior pituitary hormone secretion. Acta Endocrinol (Copenh) 129:489–496
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660
Korbonits M, Kaltsas G, Perry LA, Putignano P, Grossman AB, Besser GM, Trainer PJ (1999a) The growth hormone secretagogue hexarelin stimulates the hypothalamo-pituitary-adrenal axis via arginine vasopressin. J Clin Endocrinol Metab 84:2489–2495
Korbonits M, Little JA, Forsling ML, Tringali G, Costa A, Navarra P, Trainer PJ, Grossman AB (1999b) The effect of growth hormone secretagogues and neuropeptide Y on hypothalamic hormone release from acute rat hypothalamic explants. J Neuroendocrinol 11:521–528
Lennard DE, Eckert WA, Merchenthaler I (1993) Corticotropin-releasing hormone neurons in the paraventricular nucleus project to the external zone of the median eminence: a study combining retrograde labeling with immunocytochemistry. J Neuroendocrinol 5:175–181
Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW (2001) Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA 98:7570–7575
Mozid AM, Tringali G, Forsling ML, Hendricks MS, Ajodha S, Edwards R, Navarra P, Grossman AB, Korbonits M (2003) Ghrelin is released from rat hypothalamic explants and stimulates corticotrophin-releasing hormone and arginine-vasopressin. Horm Metab Res 35:455–459
Nakagawa T, Ukai K, Ohyama T, Koida M, Okamura H (1996) Effects of the synthesized growth hormone releasing peptide, KP-102, on growth hormone release in sodium glutamate monohydrate-treated low growth rats. Life Sci 59:705–712
Okada K, Ishii S, Minami S, Sugihara H, Shibasaki T, Wakabayashi I (1996) Intracerebroventricular administration of the growth hormone-releasing peptide KP-102 increases food intake in free-feeding rats. Endocrinology 137:5155–5158
Oki Y, Nicholson WE, Orth DN (1990) Role of protein kinase-C in the adrenocorticotropin secretory response to arginine vasopressin (AVP) and the synergistic response to AVP and corticotropin-releasing factor by perfused rat anterior pituitary cells. Endocrinology 127:350–357
Oliver C, Conte-Devolx B, Giraud P, Gillioz P, Lissitzky JC, Boudouresque F (1980) Hypothalamic control of ACTH secretion. Horm Res 13:230–241
Pelleymounter MA, Joppa M, Ling N, Foster AC (2004) Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides 25:659–666
Raadsheer FC, Sluiter AA, Ravid R, Tilders FJ, Swaab DF (1993) Localization of corticotropin-releasing hormone (CRF) neurons in the paraventricular nucleus of the human hypothalamus; age-dependent colocalization with vasopressin. Brain Res 615:50–62
Raun K, Hansen BS, Johansen NL, Thogersen H, Madsen K, Ankersen M, Andersen PH (1998) Ipamorelin, the first selective growth hormone secretagogue. Eur J Endocrinol 139:552–561
Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW and Sawchenko PE (2001) Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA 98:2843–2848
Rivier C, Vale W (1983) Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology 113:939–942
Smith RG, Van der Ploeg LH, Howard AD, Feighner SD, Cheng K, Hickey GJ, Wyvratt MJ Jr, Fisher MH, Nargund RP, Patchett AA (1997) Peptidomimetic regulation of growth hormone secretion. Endocr Rev 18:621–645
Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K (2000) Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab 85:4908–4911
Thomas GB, Fairhall KM, Robinson IC (1997) Activation of the hypothalamo-pituitary-adrenal axis by the growth hormone (GH) secretagogue, GH-releasing peptide-6, in rats. Endocrinology 138:1585–1591
Turkelson CM, Arimura A, Culler MD, Fishback JB, Groot K, Kanda M, Luciano M, Thomas CR, Chang D, Chang JK, Shimizu M (1981) In vivo and in vitro release of ACTH by synthetic CRF. Peptides 2:425–429
Wren AM, Small CJ, Fribbens CV, Neary NM, Ward HL, Seal LJ, Ghatei MA, Bloom SR (2002) The hypothalamic mechanisms of the hypophysiotropic action of ghrelin. Neuroendocrinology 76:316–324
Wu D, Chen C, Katoh K, Zhang J, Clarke IJ (1994) The effect of GH-releasing peptide-2 (GHRP-2 or KP 102) on GH secretion from primary cultured ovine pituitary cells can be abolished by a specific GH-releasing factor (GRF) receptor antagonist. J Endocrinol 140:R9–R13
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirotani, C., Oki, Y., Ukai, K. et al. ACTH releasing activity of KP-102 (GHRP-2) in rats is mediated mainly by release of CRF. Naunyn-Schmiedeberg's Arch Pharmacol 371, 54–60 (2005). https://doi.org/10.1007/s00210-004-1009-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-004-1009-3