Abstract
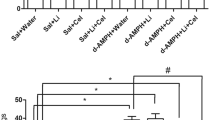
The effect of catechol-O-methyltransferase (COMT) deficiency on methamphetamine-induced hydroxyl radical production in the brain was assessed by the salicylate trapping method. Methamphetamine-induced hyperthermia was also studied. Furthermore, the effect of COMT deficiency on the activities of glutathione S-transferase, quinone reductase and liver mono-oxygenases was assessed with and without l-dopa challenge. Finally, two alternative pathways of l-dopa metabolism were evaluated. Methamphetamine increased 2,3-dihydroxybenzoic acid levels only slightly (n.s.) at the lowest dose level (2.5 mg/kg × 4 i.p.). This was accompanied by a simultaneous increase in salicylate levels so that the 2,3-dihydroxybenzoic acid/salicylate ratio decreased correspondingly. Most importantly, no COMT genotype-dependent changes were observed. However, hyperthermia was induced even at the lowest methamphetamine dose, the COMT-deficient mice being most sensitive. COMT deficiency did not significantly change the activities of liver glutathione S-transferase, quinone reductase or 7-ethoxyresorufin and 7-pentoxyresorufin O-dealkylation. In COMT-deficient female mice, l-dopa (30–80 mg/kg b.i.d. for 2 days) did not induce any significant changes in liver or brain glutathione S-transferase and quinone reductase activity or liver 7-ethoxyresorufin O-deethylation activity. The levels of l-dopa conjugates in urine were also negligible in COMT-deficient mice. Skin tyrosinase activity was increased in 7- to 8-day-old hairless COMT-deficient pups. The present results suggest that despite the increased hyperthermic response, COMT deficiency does not increase methamphetamine-induced hydroxyl radical production or change significantly the activity of certain enzymes involved in defense against reactive oxygen species. In conclusion, we found no evidence of increased oxidative stress in the liver or brain of adult mice lacking COMT activity.

Similar content being viewed by others
References
Albers DS, Sonsalla PK (1995) Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther 275:1104–1114
Asanuma M, Miyazaki I, Ogawa N (2003) Dopamine- or l-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox Res 5:165–176
Barouki R, Morel Y (2001) Repression of cytochrome P450 1A1 gene expression by oxidative stress: mechanisms and biological implications. Biochem Pharmacol 61:511–516
Basma AN, Morris EJ, Nicklas WJ, Geller HM (1995) l-DOPA cytotoxicity to PC12 cells in culture is via its autoxidation. J Neurochem 64:825–832
Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W Jr, Holson RR (1994) Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther 268:1571–1580
Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparanta T, Mayer RT (1985) Ethoxy-, pentoxy- and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem Pharmacol 34:3337–3345
Camp DM, Loeffler DA, LeWitt PA (2000) l-DOPA does not enhance hydroxyl radical formation in the nigrostriatal dopamine system of rats with a unilateral 6-hydroxydopamine lesion. J Neurochem 74:1229–1240
Davidson C, Gow AJ, Lee TH, Ellinwood EH (2001) Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Rev 36:1–22
Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD (1989) Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem 52:1830–1836
Duffy S, So A, Murphy TH (1998) Activation of endogenous antioxidant defenses in neuronal cells prevents free radical-mediated damage. J Neurochem 71:69–77
Dupont I, Berthou F, Bodenez P, Bardou L, Guirriec C, Stephan N, Dreano Y, Lucas D (1999) Involvement of cytochromes P-450 2E1 and 3A4 in the 5-hydroxylation of salicylate in humans. Drug Metab Dispos 27:322–326
Fahn S (1997) Levodopa-induced neurotoxicity: does it represent a problem for the treatment of Parkinson’s disease? CNS Drugs 8:376–393
Fleckenstein AE, Wilkins DG, Gibb JW, Hanson GR (1997) Interaction between hyperthermia and oxygen radical formation in the 5-hydroxytryptaminergic response to a single methamphetamine administration. J Pharmacol Exp Ther 283:281–285
Fogassy E, Ács M, Faigl F, Simon K, Rohonczy J, Ecsery Z (1986) Pseudosymmetry and chiral discrimination in optical resolution via diastereoisomeric salt formation. The crystal structures of (R)- and (S)-N-methylamphetamine bitartrates (RMERTA and SMERTA). J Chem Soc Perkin Trans 2:1881–1886
Gerlach M, Xiao AY, Kuhn W, Lehnfeld R, Waldmeier P, Sontag KH, Riederer P (2001) The central catechol-O-methyltransferase inhibitor tolcapone increases striatal hydroxyl radical production in l-DOPA/carbidopa treated rats. J Neural Transm 108:189–204
Giovanni A, Liang LP, Hastings TG, Zigmond MJ (1995) Estimating hydroxyl radical content in rat brain using systemic and intraventricular salicylate: impact of methamphetamine. J Neurochem 64:1819–1825
Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M (1998) Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 95:9991–9996
Haasio K, Huotari M, Nissinen E, Männistö PT (2003) Tissue histopathology, clinical chemistry and behaviour of adult Comt-gene-disrupted mice. J Appl Toxicol 23:213–219 DOI 10.1002/jat.909
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Halliwell B (1992) Reactive oxygen species and the central nervous system. J Neurochem 59:1609–1623
Halliwell B, Kaur H, Ingelman-Sundberg M (1991) Hydroxylation of salicylate as an assay for hydroxyl radicals: a cautionary note. Free Radic Biol Med 10:439–441
Huotari M, Gogos JA, Karayiorgou M, Koponen O, Forsberg M, Raasmaja A, Hyttinen J, Männistö PT (2002) Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. Eur J Neurosci 15:246–256
Huotari M, Garcia-Horsman JA, Karayiorgou M, Gogos JA, Männistö PT (2004) d-Amphetamine responses in catechol-O-methyltransferase (COMT) disrupted mice. Psychopharmacology (Berl) 172:1–10 DOI 10.1007/s00213-003-1627-3
Ingelman-Sundberg M, Kaur H, Terelius Y, Persson JO, Halliwell B (1991) Hydroxylation of salicylate by microsomal fractions and cytochrome P-450. Lack of production of 2,3-dihydroxybenzoate unless hydroxyl radical formation is permitted. Biochem J 276:753–757
Jenner P, Olanow CW (1996) Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 47 [Suppl 3]:S161–S170
Kita T, Paku S, Takahashi M, Kubo K, Wagner GC, Nakashima T (1998) Methamphetamine-induced neurotoxicity in BALB/c, DBA/2N and C57BL/6N mice. Neuropharmacology 37:1177–1184
Kitamura S, Tatsumi K (1997) Purification of NADPH-linked and NADH-linked quinone reductases from liver cytosol of sea bream, Pagrus major. Comp Biochem Physiol B Biochem Mol Biol 118:675–680
Kondo T, Ito T, Sugita Y (1994) Bromocriptine scavenges methamphetamine-induced hydroxyl radicals and attenuates dopamine depletion in mouse striatum. Ann NY Acad Sci 738:222–229
Kopin I (1985) Catecholamine metabolism: basic aspects and clinical significance. Pharmacol Rev 37:333–364
Kuhn W, Woitalla D, Gerlach M, Russ H, Müller T (1998) Tolcapone and neurotoxicity in Parkinson’s disease. Lancet 352:1313–1314
Lyras L, Zeng B-Y, McKenzie G, Pearce RKB, Halliwell B, Jenner P (2002) Chronic high dose l-DOPA alone or in combination with the COMT inhibitor entacapone does not increase oxidative damage or impair the function of the nigro-striatal pathway in normal cynomologus monkeys. J Neural Transm 109:53–67 DOI 10.1007/s702-002-8230
Martínez JH, Solano F, Arocas A, García-Borrón JC, Iborra JL, Lozano JA (1987) The existence of apotyrosinase in the cytosol of Harding-Passey mouse melanoma melanocytes and characteristics of enzyme reconstitution by Cu(II). Biochim Biophys Acta 923:413–420
Männistö PT, Kaakkola S (1999) Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev 51:593–628
McCabe DR, Maher TJ, Acworth IN (1997) Improved method for the estimation of hydroxyl free radical levels in vivo based on liquid chromatography with electrochemical detection. J Chromatogr B Biomed Sci Appl 691:23–32
Mena MA, Pardo B, Casarejos MJ, Fahn S, García de Yébenes J (1992) Neurotoxicity of levodopa on catecholamine-rich neurons. Mov Disord 7:23–31
Michel PP, Hefti F (1990) Toxicity of 6-hydroxydopamine and dopamine for dopaminergic neurons in culture. J Neurosci Res 26:428–435
Miller JW, Selhub J, Joseph JA (1996) Oxidative damage caused by free radicals produced during catecholamine autoxidation: protective effects of O-methylation and melatonin. Free Radical Biol Med 21:241–249
Morel Y, Barouki R (1999) Repression of gene expression by oxidative stress. Biochem J 342:481–496
Mytilineou C, Han SK, Cohen G (1993) Toxic and protective effects of l-DOPA on mesencephalic cell cultures. J Neurochem 61:1470–1478
Nappi AJ, Vass E (1998) Hydroxyl radical formation via iron-mediated Fenton chemistry is inhibited by methylated catechols. Biochim Biophys Acta 1425:159–167
Olanow CW (1990) Oxidation reactions in Parkinson’s disease. Neurology 40 [Suppl 3]:32–37
Olanow CW, Tatton WG (1999) Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci 22:123–144
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2378
Pearce RE, McIntyre CJ, Madan A, Sanzgiri U, Draper AJ, Bullock PL, Cook DC, Burton LA, Latham J, Nevins C, Parkinson A (1996) Effects of freezing, thawing, and storing human liver microsomes on cytochrome P450 activity. Arch Biochem Biophys 331:145–169
Raza H, Robin M-A, Fang J-K, Avadhani NG (2002) Multiple isoforms of mitochondrial glutathione S-transferases and their differential induction under oxidative stress. Biochem J 366:45–55 DOI 10.1042/BJ20020533
Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB (1989) Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 52:515–520
Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD (1994) Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol 36:348–355
Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds GP, Hebenstreit G, Youdim MB (1988) Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm 74:199–205
Sofic E, Lange KW, Jellinger K, Riederer P (1992) Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson’s disease. Neurosci Lett 142:128–130
Valverde P, García-Borrón JC, Martínez-Liarte JH, Solano F, Lozano JA (1992) Melanocyte stimulating hormone activation of tyrosinase in B16 mouse melanoma cells. Evidence for a differential induction of two distinct isoenzymes. FEBS Lett 304:114–118
Valverde P, García-Borrón JC, Jimenez-Cervantes C, Solano F, Lozano JA (1993) Tyrosinase isoenzymes in mammalian melanocytes. II. Differential activation by α-melanocyte-stimulating hormone. Eur J Biochem 217:541–548
Winder AJ (1994) A stopped spectrophotometric assay for the dopa oxidase activity of tyrosinase. J Biochem Biophys Methods 28:173–183
Acknowledgements
The authors wish to thank Dr Ewen MacDonald for revision of the manuscript. We also appreciate the excellent technical assistance of Mrs Jaana Leskinen and Ms Terttu Jokinen. This work was supported in part by grants from the Finnish Cultural Foundation of Northern Savo, the Finnish Parkinson Foundation, the Association of Finnish Pharmacies and the Finnish Pharmacists’ Association (to M.M.F.) and the Academy of Finland (to P.T.M., no. 50324).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forsberg, M.M., Juvonen, R.O., Helisalmi, P. et al. Lack of increased oxidative stress in catechol-O-methyltransferase (COMT)-deficient mice. Naunyn-Schmiedeberg's Arch Pharmacol 370, 279–289 (2004). https://doi.org/10.1007/s00210-004-0967-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-004-0967-9