Abstract
Primary human hepatocytes are used in all facets of liver research, from in vitro studies of xenobiotic disposition and toxicity to the clinical management of liver failure. Unfortunately, cellular stress during isolation and cryopreservation causes a highly unpredictable loss of the ability to attach and form cell–matrix and cell–cell interactions. Reasoning that this problem could be mitigated at the post-thawing stage, we applied label-free quantitative global proteomics to analyze differences between attached and non-attached fractions of cryopreserved human hepatocyte batches. Hepatocytes that were unable to attach to a collagen matrix showed many signs of cellular stress, including a glycolytic phenotype and activation of the heat shock response, ultimately leading to apoptosis activation. Further analysis of the activated stress pathways revealed an increase in early apoptosis immediately post-thawing, which suggested the possibility of stress reversal. Therefore, we transiently treated the cells with compounds aimed at decreasing cellular stress via different mechanisms. Brief exposure to the pan-caspase apoptosis inhibitor Z-VAD-FMK restored attachment ability and promoted a differentiated morphology, as well as formation of 3D spheroids. Further, Z-VAD-FMK treatment restored metabolic and transport functions, with maintained sensitivity to hepatotoxic insults. Altogether, this study shows that differentiation and function of suboptimal human hepatocytes can be restored after cryopreservation, thus markedly increasing the availability of these precious cells.
Similar content being viewed by others
Introduction
Primary human hepatocytes are essential to the study of liver biology and function in health and disease. Primary hepatocytes in various culture formats are considered the “gold standard” model for studies of xenobiotic metabolism, transport, and toxicity (Pedersen et al. 2013; Sison-Young et al. 2017; Vildhede et al. 2015). Hepatocytes are commonly used in two- and three-dimensional formats that require cell attachment, including sandwich cultures, spheroid aggregates, co-cultures with supportive cells, and microfluidic liver-on-a-chip platforms (Bell et al. 2016; Godoy et al. 2013; Khetani and Bhatia 2008; Swift et al. 2010; Vernetti et al. 2016; Vildhede et al. 2016).
Unfortunately, cell isolation causes a partial loss of attachment ability, with unpredictable batch-to-batch variability (Gramignoli et al. 2014). Freshly isolated hepatocytes are not available on a regular basis, and researchers therefore rely upon cryopreservation to ensure a steady hepatocyte supply (Godoy et al. 2013). Cryopreservation, which is used for long-term storage of human hepatocytes, can further aggravate the damages induced by isolation (Stéphenne et al. 2010). Modern cryopreservation is a result of decades of research into hypothermic storage solutions, cryoprotectants, optimal cooling rates, and thawing protocols (Hewitt 2010; Stéphenne et al. 2010). Although this has enabled the use of cryopreserved hepatocytes for many applications where only fresh cells were trusted in the past (Hewitt and Li 2015; Khetani et al. 2015), the variability in quality between different cryopreserved hepatocyte batches remains a problem (Khetani et al. 2015).
Cell–cell and cell–extracellular matrix (ECM) interactions promote hepatocyte differentiation and function (Ben-Ze’ev et al. 1988). Therefore, an important measure of batch quality is its attachment efficiency, defined as the percentage of hepatocytes in a batch that can attach to a surface coated with ECM proteins (typically type I collagen). A related parameter is the monolayer confluence, i.e. the percentage of a surface covered by cells, which is often used for simple quality classification of hepatocyte batches. Cryopreserved hepatocyte batches show variable attachment efficiency and confluence (Alexandre et al. 2012). Previous strategies to reduce this problem have focused on modifying the conditions prior to or during cryopreservation. Pre-incubating the cells for a short time before cryopreservation has been shown to improve attachment efficiency post-thawing (Silva et al. 1999). Supplementing the pre-incubation medium with antioxidants or other stress-reducing compounds can provide further benefit (Terry et al. 2006; Yagi et al. 2001). Alternatively, DMSO can be combined with other cryoprotectants, such as trehalose, during the cooling process itself (Katenz et al. 2007). These approaches have shown some merit, but less attention has been given to factors that could restore attachment efficiency and important hepatocyte functions after thawing.
Here, we analyzed the proteomes of attached and non-attached fractions of cryopreserved human hepatocyte batches of different quality post-thawing. Cells that failed to attach were markedly affected by cellular stress, and showed increased apoptosis activation. Interestingly, we found that early, but not late, apoptosis markers were elevated immediately after thawing, suggesting that the stress could be reversible. We therefore developed a simple anti-apoptosis protocol, where a brief exposure to the caspase inhibitor Z-VAD-FMK improved attachment and differentiated functionality of suboptimal hepatocyte batches, while the effects of compounds modulating other stress-related pathways were limited. We conclude that our simple apoptosis inhibition protocol can considerably improve the morphological and functional properties of cryopreserved human hepatocytes. This should be useful for all types of studies that use human hepatocytes, as cell attachment and morphological differentiation are fundamental requirements for most culture formats and applications.
Materials and methods
Materials
Cell culture media and supplements were purchased from Sigma-Aldrich or Thermo Fisher Scientific, unless otherwise indicated. Compounds were obtained from Sigma-Aldrich, Abcam, UBP Bio, LGC Standards, and AK Scientific.
Hepatocyte isolation
Primary hepatocytes were isolated from excess liver tissue from human donors undergoing surgical liver resection at the Department of Surgery, Uppsala University Hospital, using a two-step collagenase perfusion technique described elsewhere (LeCluyse and Alexandre 2010). Informed consent was obtained from all donors, in agreement with the approval from the Uppsala Regional Ethical Review Board (Ethical Approval no. 2009/028).
Cryopreservation
Hepatocytes were cryopreserved at 10 × 106 viable cells per cryovial, and stored at − 150 °C. Vials were thawed by immersion in a 37 °C water bath for 2 min, followed by density centrifugation to remove dead cells.
Hepatocyte culture
Thawed hepatocytes were resuspended in warm suspension and attachment medium (SAM) (LeCluyse and Alexandre 2010). Cell number, size, and viability were determined with image cytometry. Cells were seeded in collagen I-coated multiwell plates at 0.75 × 106 cells/ml and incubated at 37 °C with 5% CO2. After 3 h, the medium was removed and replaced with supplemented, serum-free Hepatocyte Maintenance Medium (Lonza; complete HMM). Attachment efficiency was calculated by counting non-attached cells in culture supernatants. For spheroid culture, thawed hepatocytes were seeded in 384-well ultra-low attachment plates (Corning) at 5000 cells/well and incubated as above. After 48 h, 80% of the medium was exchanged for complete HMM, which was repeated every 48–72 h up to 21 days.
Monolayer assessment
Images of hepatocyte monolayers after 24 h in culture were assigned a ‘monolayer score’ of 1–4 (4 being the best), representing an overall morphological assessment of its quality (Fig. 2c). Morphological parameters were included due to the importance of close cell–cell interactions and uniform, polygonal shapes for the maintenance of a hepatic phenotype (Arterburn et al. 1995; Ben-Ze’ev et al. 1988).
Proteomic analysis
Hepatocytes were cultured as described above. After 3 h, non-attached cells were collected by aspiration. Attached cells were scraped in ice-cold DPBS. Proteins were extracted with a buffer containing 2% SDS. Samples were processed with the multi-enzyme digestion filter-aided sample preparation (MED-FASP) protocol, using Lys-C and trypsin (Wiśniewski and Mann 2012). Total protein and peptide contents were measured with a tryptophan fluorescence assay (Wiśniewski and Gaugaz 2015). Proteomic analysis was performed with LTQ Orbitrap and Q Exactive HF mass spectrometers (Thermo Fisher Scientific). MS data was analyzed with MaxQuant (Cox and Mann 2008). Proteins were identified by searching MS and MS/MS data of peptides against a decoy version of the UniProtKB (May 2013). Protein concentrations were calculated using the Total Protein Approach (Wiśniewski and Rakus 2014). The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Vizcaíno et al. 2015) partner repository with the dataset identifier PXD010533.
Bioinformatic analysis
Proteomic datasets were processed in Perseus, version 1.5.0.15, retaining proteins that were detected in at least 60% of both attached and non-attached hepatocyte samples. In the LTQ Orbitrap dataset, differently expressed proteins were identified in a paired t test with the Ptest tool (Camargo et al. 2008), using a permutation-based false discovery rate of 0.05, based on 10,000 random permutations. Significantly different proteins were subjected to pathway analysis using the Reactome database, version 57 (Fabregat et al. 2016). The Q Exactive HF data was similarly processed, but using a t test as implemented in the Perseus software.
Analysis of oxidative stress and apoptosis
Oxidative stress was analyzed via dihydroethidium (DHE) fluorescence. Apoptosis was analyzed using fluorescent markers for activated caspase-8 and -3 with CaspGLOW Fluorescein Active Caspase Staining Kits (BioVision), and for phosphatidylserine externalization with an annexin V-FITC conjugate (BioLegend).
Imaging of apoptosis activation in plated hepatocytes
Cultured hepatocytes were stained for activated caspase-8 with FITC-IETD-FMK (BioVision). Membranes were stained with wheat germ agglutinin (WGA) conjugated to Alexa Fluor 555 (Thermo Fisher Scientific). Nuclei were stained with DAPI (Thermo Fisher Scientific). Samples were imaged with an LSM 700 confocal microscope (Carl Zeiss).
Inhibition of non-attachment
Hepatocytes were seeded in collagen I-coated 24-well plates (500 µl/well). 2.5 µl of DMSO stocks of a series of selected compounds were added to the culture medium (Fig. 5a), and 2.5 µl DMSO was added to control wells. The cells were allowed to attach for 3 h, and attachment efficiency was calculated as described above.
Hepatocyte function after treatment with Z-VAD-FMK
During the first 3 h, hepatocyte monolayer cultures were treated with 19 µM Z-VAD-FMK or DMSO vehicle. Medium was replaced with complete HMM, and the cells were cultured for 24 h. The transient Z-VAD-FMK treatment did not leave quantifiable amounts of the compound at the time of the experiments. In spheroids, the cells were treated with 19 µM Z-VAD-FMK during the initial 48 h. Metabolic activity was studied with diclofenac and midazolam, and uptake transport was studied with pitavastatin. Compounds were quantified with UPLC–MS/MS. Albumin secretion was measured by ELISA. Chlorpromazine and celecoxib were used to assess drug toxicity, by measuring ATP levels and caspase activities.
Detailed descriptions of the methods are provided in the Supplementary Material.
Results
Post-thaw quality assessment of cryopreserved human hepatocytes
Cryopreserved human hepatocyte batches from 14 different donors (see Table S1 for donor demographics), selected to cover a wide quality range, were characterized for standard post-thaw properties reported by major commercial vendors (Fig. 1). Viability should be in the range of 80–90% or above (Hewitt and Li 2015). Our 14 batches spanned 78.1–93.5% with an average of 89 ± 4% (Fig. 2a). Attachment-dependent confluence is commonly used to qualify batches for use in applications requiring cell attachment, with thresholds at e.g. 70 or 80% (Alexandre et al. 2012; Li 2015). The attachment efficiency of our 14 batches ranged between 55.1–95.1%, and confluence, defined as the percentage of a surface covered by cells, spanned a similar range of 59.7–100% (Fig. 2b). Despite the strong correlation with attachment efficiency (Pearson’s r = 0.90), confluence is in our experience an insufficient classifier of batch quality, as monolayers from different allegedly attachment-qualified batches can show highly variable morphology.
Assessment of post-thaw quality of 14 cryopreserved human hepatocyte batches. a Cell viability immediately after thawing. b Attachment efficiency after 3 h in culture, and monolayer confluence at 24 h. c Batches were divided into three groups based on monolayer score assessments (heplow, hepmid, and hephigh). d Representative examples of monolayers from the three groups after 24 h in culture. Images were captured at ×10 magnification
We therefore developed a ‘monolayer score’ that contained an assessment of the hepatocyte morphology. The morphological evaluation included cell shape, cell stretching, cell–cell interactions, and the presence of dead cells and debris. Confluence influenced the monolayer score positively, whereas atypical cellular morphology had a negative influence (Fig. 2c), further confirmed by PLS modeling (Fig. S1). In summary, the monolayer score offered a more holistic view of cell quality and was used to classify hepatocyte batches in this work. After scoring, the batches were evenly divided into three groups: heplow (monolayer score < 2; n = 5), hepmid (monolayer score 2–3; n = 5) and hephigh (monolayer score > 3; n = 4; Fig. 2c, d).
Global proteomic analysis of attached and non-attached hepatocytes
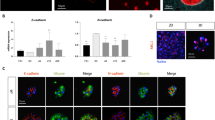
Next, attached and non-attached hepatocyte fractions were collected from individual batches after 3 h in culture and subjected to quantitative global proteomic analysis. We identified 4013 proteins in matched samples from nine batches (including representatives from each monolayer score group; Dataset 1). 3237 proteins were detected in at least 60% of both sample groups and considered for further investigation (Fig. S2A). Principal component analysis of the proteomic data indicated pronounced differences in protein expression between hepatocytes from the attached and non-attached fractions (Fig. 3a). Thus, 896 proteins (27.7% of considered proteins) had significantly different concentrations (adjusted P < 0.05; Fig. 3b; Fig. S2A).
Global proteomic analysis of attached and non-attached hepatocyte fractions. a Principal component analysis of the proteomic data. The numbers in parentheses show the variability explained by each component. b 9.2% and 18.4% of the analyzed proteins were present at significantly different levels in the attached and non-attached fractions, respectively. c Biological pathways and functions that were activated in the different fractions. d Schematic representation of the heat shock response, which was activated in the non-attached fractions
Pathway analysis revealed activation of distinct biological pathways and functions in attached and non-attached hepatocytes (Fig. 3c; Table S2, and Table S3). Notably, marked activation of apoptosis was observed in the non-attached fractions, and there were differences in energy metabolism. The non-attached fractions showed a glycolytic phenotype typical for stressed cells, whereas the attached fractions obtained energy via lipid metabolism and oxidative phosphorylation, which more resembles the situation in the normal liver (Soboll 1995). Further, metabolism of amino acids, nucleotides (especially purines), and proteins was increased in the non-attached fractions. We also specifically looked at abundances of key adhesion proteins, i.e. integrins, adherens junction proteins, and tight junction proteins, to investigate their role in non-attachment. Attached and non-attached hepatocyte fractions showed very similar levels of these proteins, which suggests that altered subcellular distribution of adhesion proteins, most likely in plasma membrane concentrations, rather than altered abundances, was potentially involved in cell attachment differences (Fig. S2B). This could occur through stress-induced endocytosis of the plasma membrane (Lin et al. 2013).
To obtain deeper proteome coverage, replicate samples of attached and non-attached fractions from two batches (L5 and H4) were analyzed at higher resolution. Here, 7008 proteins were identified (Dataset 2), including more than 93% of the previous data (Fig. S2C–S2E). The data was processed as above, and 2559 proteins were present at significantly different levels. The results were in good agreement with those described above, but the cellular response to heat stress was additionally identified as an enriched pathway in the non-attached fractions (Fig. S2F, Table S4, and Table S5). This important protective mechanism reacts to various types of cell stress, including oxidative stress (Kültz 2005), which if left unresolved eventually triggers cell death pathways (Fig. 3d).
Involvement of apoptosis in poor monolayer formation
Based on the proteomic observations, we performed more detailed analysis of oxidative stress and apoptosis. Oxidative stress levels showed a 4.3-fold increase in the non-attached fractions compared to the attached fractions (P = 0.0007), providing a likely trigger for apoptosis activation (Fig. 4a). Indeed, heplow batches, which contained large numbers of non-attached cells, showed significantly elevated caspase activation immediately after thawing (Fig. 4b). Interestingly, phosphatidylserine externalization (annexin V binding) was only marginally increased, indicating that most cells had not reached this later stage of apoptosis (Fig. 4b). After 3 h in culture, however, the non-attached fractions from representative batches (see Table S6 for batch use) showed a threefold rise in annexin V binding compared to post-thaw levels (P = 0.0018; Fig. 4c), indicating apoptosis progression accompanied by cell shrinkage of more than 1 µm in diameter (Fig. 4d). In addition, high caspase activity was observed in the attached fraction of a heplow batch (Fig. 4e). Due to ongoing cell death, the non-attached fractions of all batches showed a decreased mean viability to 75 ± 4%, compared to 89 ± 4% immediately post-thawing (P < 0.0001; Fig. 4f).
Cell stress and apoptosis in cryopreserved human hepatocytes at various stages after thawing. a Oxidative stress levels in attached and non-attached cell fractions. ***P < 0.001, Student’s t test. b Apoptosis levels immediately after thawing, assessed by caspase-3 and -8 activity, and phosphatidylserine externalization (via annexin V binding). **P < 0.01, ***P < 0.001, ****P < 0.0001, two-way ANOVA with Tukey’s multiple comparisons test. c Annexin V binding after thawing and in the non-attached fractions, measured in a subset of batches. **P < 0.01, Student’s t test. d Average cell size distributions in post-thaw and non-attached hepatocytes. e Caspase-8 activation in attached cells from three representative batches. f Cell viability after thawing and in the non-attached fractions after 3 h in culture
Improving attachment efficiency and monolayer morphology
We next considered apoptosis and six other biological mechanisms whose perturbation could potentially decrease cell stress and lead to increased attachment efficiency. These included activation of AMP-activated protein kinase (AMPK), autophagy inhibition, blocking of massive endocytosis (MEND), stimulation of mitochondrial fusion, necroptosis inhibition, and reactive oxygen species (ROS) inhibition (Fig. 5a). We selected 16 compounds targeted at these mechanisms (Fig. 5a) and investigated to what extent a cryopreserved hepatocyte batch (from the heplow group; batch L5) could be ‘rescued’. In general, compound concentrations were chosen to cover a tenfold range around a value with previously documented effect on the mechanism of interest (Table S7). The largest increase in attachment efficiency was observed after apoptosis inhibition with the pan-caspase inhibitor Z-VAD-FMK (significant increase over other compounds, one-way ANOVA; Fig. 5b). Z-VAD-FMK also gave a markedly improved monolayer score, moving the L5 batch from the heplow group to the hephigh group (an increase from 1.3 to 3.3 at 19 µM), effectively making it a high quality batch (Fig. 5b, c; Fig. S3).
Improving attachment efficiency and monolayer morphology of cryopreserved human hepatocytes after thawing. a Mechanisms to perturb for potential improvement of cell attachment and morphology, and the corresponding compounds used. b Attachment efficiency of heplow (L5) cells treated with low and high doses of each compound, expressed as a percentage of the corresponding DMSO control. The most successful compound, the pan-caspase inhibitor Z-VAD-FMK, is encircled in gray. Symbol colors represent the monolayer scores of the ensuing monolayers. c Monolayers from hepatocytes (L5) cultured with DMSO or 19 µM (low dose) Z-VAD-FMK. d Attachment efficiency of four hepatocyte batches of different qualities, treated with a series of Z-VAD-FMK concentrations. Line colors show the original monolayer score group of the batches, and the colored dots show the monolayer classification at 0 and 19 µM Z-VAD-FMK. e Hepatocyte spheroids after 21 days in culture, cultured with or without 19 µM Z-VAD-FMK during the first 48 h. f Principal component analysis of global proteomic data from hepatocytes treated with DMSO or 19 µM Z-VAD-FMK for 3 h. Samples were collected after 3 and 24 h in culture. The numbers in parentheses show the variability explained by each component. Error bars show standard deviations from two independent experiments. (Color figure online)
We then measured the concentration-dependent effect of Z-VAD-FMK (0.019–190 µM) in four hepatocyte batches of different qualities (L3, L5, M1, and H3). For heplow batches, the addition of Z-VAD-FMK resulted in a remarkable concentration-dependent increase in attachment efficiency (Fig. 5d). At 19 µM, attachment efficiency of the batch with the lowest monolayer score (L3) was increased by over 1.5-fold, from 39.5 ± 8.1% to 61.5 ± 5.9% (Fig. 5d). Confluence increased from 57.5 ± 10.6% to 87.5 ± 1.1%, and the monolayer score went from 1.2 to 2.3, thus approaching the hephigh group (Fig. 5d; Fig. S4). The hepmid batch (M1) was also improved by the Z-VAD-FMK treatment, and its monolayer score increased from the hepmid group to the hephigh group. Beneficial effects of Z-VAD-FMK were observed in long-term culture as well, where the compound assisted in the formation of three-dimensional hepatocyte spheroids in a 384-well format, with more rapid and compact spheroid formation by cells from a heplow batch after treatment (Fig. 5e; Fig. S5).
To investigate if short-term (3 h) exposure to Z-VAD-FMK influenced hepatocyte proteomes, representative heplow (L3) and hephigh (H2) batches were treated with 19 µM Z-VAD-FMK or DMSO vehicle. Samples were collected immediately after treatment and after 24 h in culture. 6211 proteins were detected (Dataset 3), but there were no statistically significant differences between treated and untreated cells at 3 or 24 h in culture, which was also observed with principal component analysis (Fig. 5f). We conclude that treatment with Z-VAD-FMK had the beneficial effect of improving hepatocyte attachment and morphology without undesirable acute shifts in protein expression.
Hepatocyte function after caspase inhibition
Common applications of isolated hepatocytes include studies of drug transport, metabolism, and toxicity. To investigate the effects of Z-VAD-FMK on hepatocyte function, we used prototypical substrates to investigate whether hepatic drug transport, metabolism, and toxicity were affected by the compound. To assess uptake transport, we studied solute carrier organic anion (SLCO) transporters, which are highly and partly specifically expressed in hepatocytes and important in the transport of many drugs, including statins, hepatitis C inhibitors, and anticancer drugs (Roth et al. 2012). Pitavastatin was selected as a substrate, as it is transported by several clinically relevant members of the SLCO family (Giacomini et al. 2010; Roth et al. 2012) and shows limited metabolism (Elsby et al. 2012). Diclofenac and midazolam were used to study metabolism via the cytochrome P450 (CYP) enzymes CYP2C9 and CYP3A4, respectively. These are two of the most important enzymes in the detoxification of xenobiotics in the liver (Zanger and Schwab 2013). General toxicity was assessed with the commonly used hepatotoxic reference compound chlorpromazine (Selim and Kaplowitz 1999). To investigate if hepatocytes treated with Z-VAD-FMK became less sensitive to toxicity requiring caspase activation, we also exposed the cells to the known apoptosis inducer celecoxib (Kern et al. 2006).
Z-VAD-FMK treatment increased pitavastatin transport capacity by more than 1.5-fold in heplow (L3) and hepmid (M1) batches (Fig. 6a; Table S8). Diclofenac clearance showed a twofold increase in the heplow batch after treatment, while midazolam clearance was increased by close to 2- and 1.5-fold in the heplow and hepmid batches, respectively (Fig. 6b; Table S9). No notable effect of Z-VAD-FMK treatment was observed in hephigh batches (H2 and H3). The appearance of major metabolites of diclofenac and midazolam (1-OH-diclofenac, 1-OH-midazolam, and 4-OH-midazolam) showed similar patterns (Table S10). After correcting for attachment efficiency, we noticed that the apparent increases in function mainly resulted from larger numbers of cells capable of attachment after Z-VAD-FMK treatment (Fig. 6a, b). On the other hand, the hepmid batch (M1) also showed some increases after this correction, suggesting that increased function in individual cells may occur in this intermediate group. We analyzed metabolism by CYP2C9 and CYP3A4 in long-term spheroid culture as well, and found similar levels of diclofenac and midazolam metabolites in treated and untreated cells (Fig. 6c). Further, albumin secretion was not affected by Z-VAD-FMK (Fig. 6d). Our proteomic data reflected the findings on hepatocyte function. Hepatocyte batches treated with Z-VAD-FMK or DMSO vehicle showed very similar expression levels of SLCO1B1, SLCO1B3, SLCO2B1, CYP2C9, and CYP3A4, as well as other CYP and UDP-glucuronosyltransferase (UGT) enzymes (Williams et al. 2004) of importance in drug metabolism (Fig. 6e–g).
Hepatocyte function after caspase inhibition with 19 µM Z-VAD-FMK. a Pitavastatin uptake transport and b diclofenac and midazolam metabolism in hepatocyte monolayers after 24 h in culture. The data is presented uncorrected and corrected for attachment efficiency. c Diclofenac and midazolam metabolism and d albumin secretion in hepatocyte spheroids cultured with or without Z-VAD-FMK. e SLCO transporter expression, f CYP enzyme expression, and g UGT enzyme expression in hepatocytes cultured with or without Z-VAD-FMK for 3 h, analyzed after 3 and 24 h in culture. h Chlorpromazine toxicity after 24 h of exposure in hepatocyte monolayers treated with DMSO or Z-VAD-FMK for 3 h. i Chlorpromazine EC50 values. j Caspase activation in hepatocyte monolayers cultured with or without Z-VAD-FMK for 3 h, induced by celecoxib exposure for 24 h. Error bars show standard deviations from two independent experiments
The toxicity of the hepatotoxic reference compound chlorpromazine was not changed by Z-VAD-FMK treatment, as shown by its EC50 values (Fig. 6h, i). Overall, the heplow batch (L3) was more sensitive to chlorpromazine, presumably due to the high oxidative stress levels and continuous apoptosis activation in hepatocytes from the heplow group. Exposure to the apoptosis inducer celecoxib gave a concentration-dependent increase in caspase activity for all batch qualities, with virtually identical responses in hepatocytes treated with Z-VAD-FMK or DMSO (Fig. 6j). In summary, Z-VAD-FMK increased the number of cells capable of attachment, and was not detrimental to hepatocyte function.
Discussion
Restoring the ability of suboptimal human hepatocyte batches to form cell–matrix and cell–cell interactions at the post-cryopreservation stage has previously been considered a futile endeavor (Hewitt and Li 2015). In this study, we show that this is not the case. Immediately after thawing, poorly performing hepatocytes displayed signs of early and reversible, rather than late and irreversible, apoptosis. Using label-free quantitative proteomics, we found that cells that failed to attach to a collagen matrix showed activation of stress response mechanisms, including apoptosis and altered energy metabolism towards a stress-related glycolytic phenotype. To abrogate the cellular stress, we modulated different stress-related pathways, and found that brief treatment with the pan-caspase inhibitor Z-VAD-FMK resulted in marked improvements in attachment efficiency, cellular morphology, and function of hepatocyte batches of suboptimal quality.
The cellular stress we observed in hepatocytes unable to attach to collagen is in agreement with the inevitably harsh isolation procedure. Oxidative stress is introduced already during surgical resection through ischemia–reperfusion injury (IRI) (Zhai et al. 2013), followed by a second reperfusion event during isolation. The isolation procedure triggers additional stress by the enzymatic detachment of cells from their in vivo architectural context (Smets et al. 2002). It has been proposed that these stressful events initiate a pre-apoptotic response, where hepatocytes are primed to enter apoptosis if subjected to further, prolonged stress (Nipič et al. 2010). Cryopreservation then causes additional oxidative as well as mechanical stress, thus exacerbating the damages induced by resection and isolation (Stéphenne et al. 2010). Cryopreservation has also been reported to downregulate important adhesion proteins, including integrin β1 and E-cadherin (Terry et al. 2007). We could not observe such differences between attached and non-attached hepatocyte fractions. However, stress-related internalization through MEND (Lin et al. 2013) and intracellular degradation of adhesion proteins to protein specific peptides, identical to those in the proteomic analysis, could provide an explanation for this. On the other hand, our proteomic data showed significant alterations in non-attached hepatocytes consistent with IRI, including increased purine metabolism (Chouchani et al. 2014) and a glycolytic phenotype (Eltzschig and Eckle 2011). This confirms that the stress resulting from IRI remained during subsequent cryopreservation and thawing. Previous studies have shown that adding antioxidants during or after isolation can partly mitigate the detrimental effects of IRI (Bhogal et al. 2010; Sagias et al. 2010), and such compounds are therefore sometimes included in current cryopreservation protocols (Hewitt and Li 2015).
Apoptosis inhibition with Z-VAD-FMK improved attachment efficiency and cellular morphology in a concentration-dependent manner. Z-VAD-FMK treatment restored a differentiated monolayer with polygonal morphology and well-defined cell–cell contacts even in batches with high attachment efficiency but suboptimal morphology (typical of the hepmid group; Fig. 2). The treatment thus appears to have rescued cells that managed to attach but would enter apoptosis later, likely as a result of cryopreservation-induced delayed-onset cell death (Baust et al. 2001). Intervention with stress-related mechanisms other than apoptosis, such as ROS inhibition or MEND block, did not give noteworthy effects on attachment efficiency and cellular morphology, suggesting that hepatocytes that fail to attach are past the point where such treatment would be useful (see Table S7 for a full list of mechanisms). Interestingly, Z-VAD-FMK achieved its effect without undesirable alterations of hepatocyte protein expression and function, suggesting that the short 3 h exposure did not interfere with normal hepatocyte function, as measured after 24 h in culture. Indeed, Z-VAD-FMK led to increased overall function in terms of uptake transport and metabolism, as many more cells were able to perform these characteristic features of differentiated hepatocytes. Beneficial effects were also seen for three-dimensional hepatocyte cultures in the principally different 384-well format, where Z-VAD-FMK allowed compact spheroids to form even with cells from a heplow batch. This shows that treated cells are well suited for long-term studies in more advanced culture formats as well.
Z-VAD-FMK did not affect the EC50 values of the hepatotoxic drug chlorpromazine. This suggested that hepatocytes treated with Z-VAD-FMK retained their sensitivity to hepatotoxic agents. However, Z-VAD-FMK is an irreversible caspase inhibitor, meaning that cellular caspases are irreversibly inhibited after treatment (Garcia-Calvo et al. 1998). This could lead to a lower sensitivity when the mechanism of toxicity involves apoptosis. Notably, our protocol only calls for a brief 3 h presence of the compound, and we found that it was washed out of the cells (was below the limit of detection). Further, global protein turnover studies have shown that caspases have a median turnover rate of less than 10 h (Boisvert et al. 2011). Since most studies of drug toxicity, or other hepatotoxic insults, entail culturing hepatocytes for at least 24 h before compound addition, we conclude that most of the cellular caspase pool has been replaced when starting the experiment. Accordingly, our brief Z-VAD-FMK treatment did not have any lingering inhibitory effects on caspase activation by the apoptosis inducer celecoxib (Fig. 6j).
In summary, we identified signs of cellular stress in the non-attached fractions of cryopreserved human hepatocyte batches. Furthermore, we found that post-thaw restoration of attachment ability and morphological differentiation in suboptimal hepatocytes is possible via apoptosis inhibition with Z-VAD-FMK, without notable proteomic changes or loss of hepatocyte function. Our simple treatment protocol can thus markedly increase the availability of well-functioning human hepatocytes that are capable of attachment, which is essential for almost all hepatocyte culture formats and applications.
References
Alexandre E, Baze A, Parmentier C et al (2012) Plateable cryopreserved human hepatocytes for the assessment of cytochrome P450 inducibility: experimental condition-related variables affecting their response to inducers. Xenobiotica 42(10):968–979
Arterburn LM, Zurlo J, Yager JD, Overton RM, Heifetz AH (1995) A morphological study of differentiated hepatocytes in vitro. Hepatology 22(1):175–187
Baust JM, Vogel MJ, Van Buskirk R, Baust JG (2001) A molecular basis of cryopreservation failure and its modulation to improve cell survival. Cell Transpl 10(7):561–571
Bell CC, Hendriks DF, Moro SM et al (2016) Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci Rep 6:25187
Ben-Ze’ev A, Robinson GS, Bucher N, Farmer SR (1988) Cell–cell and cell–matrix interactions differentially regulate the expression of hepatic and cytoskeletal genes in primary cultures of rat hepatocytes. Proc Natl Acad Sci USA 85(7):2161–2165
Bhogal RH, Curbishley SM, Weston CJ, Adams DH, Afford SC (2010) Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transpl 16(11):1303–1313
Boisvert F-M, Ahmad Y, Gierliński M et al (2011) A quantitative spatial proteomics analysis of proteome turnover in human cells. Mol Cell Proteom 11:M111.011429
Camargo A, Azuaje F, Wang H, Zheng H (2008) Permutation-based statistical tests for multiple hypotheses. Source Code Biol Med 3(1):1
Chouchani ET, Pell VR, Gaude E et al (2014) Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515(7527):431–435
Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26(12):1367–1372
Elsby R, Hilgendorf C, Fenner K (2012) Understanding the critical disposition pathways of statins to assess drug–drug interaction risk during drug development: it’s not just about OATP1B1. Clin Pharmacol Ther 92(5):584–598
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion—from mechanism to translation. Nat Med 17(11):1391–1401
Fabregat A, Sidiropoulos K, Garapati P et al (2016) The Reactome pathway knowledgebase. Nucleic Acids Res 44(D1):D481–D487
Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA (1998) Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem 273(49):32608–32613
Giacomini KM, Huang S-M, Tweedie DJ et al (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9(3):215–236
Godoy P, Hewitt NJ, Albrecht U et al (2013) Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol 87(8):1315–1530
Gramignoli R, Tahan V, Dorko K et al (2014) Rapid and sensitive assessment of human hepatocyte functions. Cell Transpl 23(12):1545–1556
Hewitt NJ (2010) Optimisation of the cryopreservation of primary hepatocytes. In: Maurel P (ed) Hepatocytes methods in molecular biology (methods and protocols), vol 640. Humana Press, New York, pp 83–105
Hewitt NJ, Li AP (2015) Cryopreservation of hepatocytes. In: Vinken M, Rogiers V (eds) Protocols in in vitro hepatocyte research methods in molecular biology (methods and protocols), vol 1250. Humana Press, New York, pp 13–26
Katenz E, Vondran FWR, Schwartlander R et al (2007) Cryopreservation of primary human hepatocytes: the benefit of trehalose as an additional cryoprotective agent. Liver Transpl 13(1):38–45
Kern MA, Haugg AM, Koch AF et al (2006) Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res 66(14):7059–7066
Khetani SR, Bhatia SN (2008) Microscale culture of human liver cells for drug development. Nat Biotechnol 26(1):120–126
Khetani SR, Berger DR, Ballinger KR, Davidson MD, Lin C, Ware BR (2015) Microengineered liver tissues for drug testing. J Lab Autom 20(3):216–250
Kültz D (2005) Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67:225–257
LeCluyse EL, Alexandre E (2010) Isolation and culture of primary hepatocytes from resected human liver tissue. In: Maurel P (ed) Hepatocytes methods in molecular biology (methods and protocols), vol 640. Humana Press, New York, pp 57–82
Li AP (2015) Evaluation of adverse drug properties with cryopreserved human hepatocytes and the integrated discrete multiple organ co-culture (IdMOCTM) system. Toxicol Res 31(2):137–149
Lin M-J, Fine M, Lu J-Y, Hofmann SL, Frazier G, Hilgemann DW (2013) Massive palmitoylation-dependent endocytosis during reoxygenation of anoxic cardiac muscle. Elife 2:e01295
Nipič D, Pirc A, Banič B, Šuput D, Milisav I (2010) Preapoptotic cell stress response of primary hepatocytes. Hepatology 51(6):2140–2151
Pedersen JM, Matsson P, Bergström CA et al (2013) Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci 136(2):328–343
Roth M, Obaidat A, Hagenbuch B (2012) OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol 165(5):1260–1287
Sagias FG, Mitry RR, Hughes RD et al (2010) N-acetylcysteine improves the viability of human hepatocytes isolated from severely steatotic donor liver tissue. Cell Transpl 19(11):1487–1492
Selim K, Kaplowitz N (1999) Hepatotoxicity of psychotropic drugs. Hepatology 29(5):1347–1351
Silva JM, Day SH, Nicoll-Griffith DA (1999) Induction of cytochrome-P450 in cryopreserved rat and human hepatocytes. Chem Biol Interact 121(1):49–63
Sison-Young RL, Lauschke VM, Johann E et al (2017) A multicenter assessment of single-cell models aligned to standard measures of cell health for prediction of acute hepatotoxicity. Arch Toxicol 91(3):1385–1400
Smets FN, Chen Y, Wang L-J, Soriano HE (2002) Loss of cell anchorage triggers apoptosis (anoikis) in primary mouse hepatocytes. Mol Genet Metab 75(4):344–352
Soboll S (1995) Regulation of energy metabolism in liver. J Bioenerg Biomembr 27(6):571–582
Stéphenne X, Sokal E, Najimi M (2010) Hepatocyte cryopreservation: is it time to change the strategy? World J Gastroenterol 16(1):1–14
Swift B, Pfeifer ND, Brouwer KL (2010) Sandwich-cultured hepatocytes: an in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metab Rev 42(3):446–471
Terry C, Dhawan A, Mitry RR, Lehec SC, Hughes RD (2006) Preincubation of rat and human hepatocytes with cytoprotectants prior to cryopreservation can improve viability and function upon thawing. Liver Transpl 12(1):165–177
Terry C, Hughes RD, Mitry RR, Lehec SC, Dhawan A (2007) Cryopreservation-induced nonattachment of human hepatocytes: role of adhesion molecules. Cell Transpl 16(6):639–647
Vernetti LA, Senutovitch N, Boltz R et al (2016) A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp Biol Med 241(1):101–114
Vildhede A, Wiśniewski JR, Norén A, Karlgren M, Artursson P (2015) Comparative proteomic analysis of human liver tissue and isolated hepatocytes with a focus on proteins determining drug exposure. J Proteome Res 14(8):3305–3314
Vildhede A, Mateus A, Khan EK et al (2016) Mechanistic modeling of pitavastatin disposition in sandwich-cultured human hepatocytes: a proteomics-informed bottom-up approach. Drug Metab Dispos 44:505–516
Vizcaíno JA, Csordas A, Del-Toro N et al (2015) 2016 update of the PRIDE database and its related tools. Nucleic Acids Res 44(D1):D447–D456
Williams JA, Hyland R, Jones BC et al (2004) Drug–drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos 32(11):1201–1208
Wiśniewski JR, Gaugaz FZ (2015) Fast and sensitive total protein and peptide assays for proteomic analysis. Anal Chem 87(8):4110–4116
Wiśniewski JR, Mann M (2012) Consecutive proteolytic digestion in an enzyme reactor increases depth of proteomic and phosphoproteomic analysis. Anal Chem 84(6):2631–2637
Wiśniewski JR, Rakus D (2014) Multi-enzyme digestion FASP and the ‘Total Protein Approach’-based absolute quantification of the Escherichia coli proteome. J Proteom 109:322–331
Yagi T, Hardin JA, Valenzuela YM, Miyoshi H, Gores GJ, Nyberg SL (2001) Caspase inhibition reduces apoptotic death of cryopreserved porcine hepatocytes. Hepatology 33(6):1432–1440
Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138(1):103–141
Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW (2013) Ischaemia–reperfusion injury in liver transplantation—from bench to bedside. Nat Rev Gastroenterol Hepatol 10(2):79–89
Acknowledgements
We thank André Mateus and Andrea Treyer for monolayer score assessments, Georgiy Khodus for assistance with confocal microscopy, Wojciech Senkowski for assistance with spheroid imaging, Katharina Zettl for technical assistance with the proteomic analysis, and Prof. Matthias Mann for continuous support. This work was supported by the Swedish Research Council, Grant nos. 2822 and 01951.
Author information
Authors and Affiliations
Contributions
MÖ and PA: conceived and designed the study. MÖ, JRW, IF, NH, and JU performed experimental work. MÖ, JRW, and PA analyzed data. MÖ and PA wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ölander, M., Wiśniewski, J.R., Flörkemeier, I. et al. A simple approach for restoration of differentiation and function in cryopreserved human hepatocytes. Arch Toxicol 93, 819–829 (2019). https://doi.org/10.1007/s00204-018-2375-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-018-2375-9