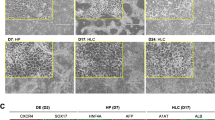
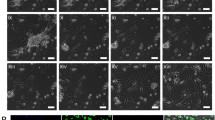
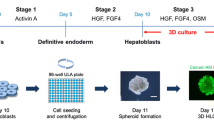
Abstract
The development of predictive in vitro stem cell-derived hepatic models for toxicological drug screening is an increasingly important topic. Herein, umbilical cord tissue-derived mesenchymal stem cells (hnMSCs) underwent hepatic differentiation using an optimized three-step core protocol of 24 days that mimicked liver embryogenesis with further exposure to epigenetic markers, namely the histone deacetylase inhibitor trichostatin A (TSA), the cytidine analogue 5-azacytidine (5-AZA) and dimethyl sulfoxide (DMSO). FGF-2 and FGF-4 were also tested to improve endoderm commitment and foregut induction during Step 1 of the differentiation protocol, being HHEX expression increased with FGF-2 (4 ng/mL). DMSO (1%, v/v) when added at day 10 enhanced cell morphology, glycogen storage ability, enzymatic activity and induction capacity. Moreover, the stability of the hepatic phenotype under the optimized differentiation conditions was examined up to day 34. Our findings showed that hepatocyte-like cells (HLCs) acquired the ability to metabolize glucose, produce albumin and detoxify ammonia. Global transcriptional analysis of the HLCs showed a partial hepatic differentiation degree. Global analysis of gene expression in the different cells revealed shared expression of gene groups between HLCs and human primary hepatocytes (hpHeps) that were not observed between HepG2 and hpHeps. In addition, bioinformatics analysis of gene expression data placed HLCs between the HepG2 cell line and hpHeps and distant from hnMSCs. The enhanced hepatic differentiation observed was supported by the presence of the hepatic drug transporters OATP-C and MRP-2 and gene expression of the hepatic markers CK18, TAT, AFP, ALB, HNF4A and CEBPA; and by their ability to display stable UGT-, EROD-, ECOD-, CYP1A1-, CYP2C9- and CYP3A4-dependent activities at levels either comparable with or even higher than those observed in primary hepatocytes and HepG2 cells. Overall, an improvement of the hepatocyte-like phenotype was achieved for an extended culture time suggesting a role of the epigenetic modifiers in hepatic differentiation and maturation and presenting hnMSC-HLCs as an advantageous alternative for drug discovery and in vitro toxicology testing.










Similar content being viewed by others
Abbreviations
- Ac-LDL:
-
Acetylated low-density lipoprotein
- AFP:
-
α-Fetoprotein
- ALB:
-
Albumin
- 5-AZA:
-
5-Azacytidine
- CK-18:
-
Cytokeratin 18
- CK-19:
-
Cytokeratin 19
- CYP:
-
Cytochrome P-450
- DMSO:
-
Dimethyl sulfoxide
- c/EBPα:
-
CCAAT/enhancer binding protein
- ECOD:
-
7-Ethoxycoumarin-O-deethylase
- EROD:
-
7-Ethoxyresorufin-O-deethylase
- FGF-2:
-
Fibroblast growth factor-2
- FGF-4:
-
Fibroblast growth factor-4
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- HHEX:
-
Hematopoietically expressed homeobox protein
- HLC:
-
Hepatocyte-like cell
- HNF-4α:
-
Hepatocyte nuclear factor-4α
- hnMSC:
-
Human neonatal mesenchymal stromal cell
- hpHep:
-
Human primary hepatocyte
- hrHep:
-
Human rat hepatocyte
- IPA:
-
Ingenuity pathway analysis
- 3-MC:
-
3-Methylcholantherene
- MRP-2:
-
Multidrug resistance protein-2
- 4-MU:
-
4-Methylumbelliferone
- OATP-C:
-
Organic anion-transporting polypeptide C
- TAT:
-
Tyrosine aminotransferase
- PCA:
-
Principal component analysis
- TSA:
-
Trichostatin A
- UCX® :
-
hnMSCs isolated from the umbilical cord matrix
- UGT:
-
Uridine 5′-diphosphate glucuronosyltransferase
References
Ameri J, Stahlberg A, Pedersen J et al (2010) FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells 28(1):45–56. doi:10.1002/stem.249
Andersson TB (2010) The application of HepRG cells in evaluation of cytochrome P450 induction properties of drug compounds. Methods Mol Biol 640:375–387. doi:10.1007/978-1-60761-688-7_20
Banas A, Teratani T, Yamamoto Y et al (2009) Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol 24(1):70–77. doi:10.1111/j.1440-1746.2008.05496.x
Brolen G, Sivertsson L, Bjorquist P et al (2010) Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J Biotechnol 145(3):284–294. doi:10.1016/j.jbiotec.2009.11.007
Buyl K, De Kock J, Najar M et al (2014) Characterization of hepatic markers in human Wharton’s jelly-derived mesenchymal stem cells. Toxicol In Vitro 28(1):113–119. doi:10.1016/j.tiv.2013.06.014
Cahan P, Li H, Morris SA, Lummertz da Rocha E, Daley GQ, Collins JJ (2014) Cell Net: network biology applied to stem cell engineering. Cell 158(4):903–915. doi:10.1016/j.cell.2014.07.020
Calder A, Roth-Albin I, Bhatia S et al (2013) Lengthened G1 phase indicates differentiation status in human embryonic stem cells. Stem Cells Dev 22(2):279–295. doi:10.1089/Scd.2012.0168
Campard D, Lysy PA, Najimi M, Sokal EM (2008) Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology 134(3):833–848. doi:10.1053/j.gastro.2007.12.024
Chetty R, Slavin JL (1994) Epithelioid sarcoma with extensive chondroid differentiation. Histopathology 24(4):400–401. doi:10.1111/j.1365-2559.1994.tb00547.x
Czysz K, Minger S, Thomas N (2015) DMSO efficiently down regulates pluripotency genes in human embryonic stem cells during definitive endoderm derivation and increases the proficiency of hepatic differentiation. PLoS ONE 10(2):e0117689. doi:10.1371/journal.pone.0117689
De Kock J, Najar M, Bolleyn J et al (2012) Mesoderm-derived stem cells: the link between the transcriptome and their differentiation potential. Stem Cells Dev 21(18):3309–3323. doi:10.1089/scd.2011.0723
Denson LA, McClure MH, Bogue CW, Karpen SJ, Jacobs HC (2000) HNF3beta and GATA-4 transactivate the liver-enriched homeobox gene, Hex. Gene 246(1–2):311–320. doi:10.1016/S0378-1119(00)00082-2
Dessimoz J, Opoka R, Kordich JJ, Grapin-Botton A, Wells JM (2006) FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech Dev 123(1):42–55. doi:10.1016/j.mod.2005.10.001
Earp HS, Rubin RA, Austin KS, Dy RC (1983) DMSO increases tyrosine residue phosphorylation in membranes from murine erythroleukemia cells. Biochem Biophys Res Commun 112(2):413–418. doi:10.1016/0006-291X(83)91479-1
Gerets HH, Tilmant K, Gerin B et al (2012) Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol 28(2):69–87. doi:10.1007/s10565-011-9208-4
Godoy P, Hewitt NJ, Albrecht U et al (2013) Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol 87(8):1315–1530. doi:10.1007/s00204-013-1078-5
Godoy P, Schmidt-Heck W, Natarajan K et al (2015) Gene networks and transcription factor motifs defining the differentiation of stem cells into hepatocyte-like cells. J Hepatol 63(4):934–942. doi:10.1016/j.jhep.2015.05.013
Godoy P, Widera A, Schmidt-Heck W et al (2016) Gene network activity in cultivated primary hepatocytes is highly similar to diseased mammalian liver tissue. Arch Toxicol. doi:10.1007/s00204-016-1761-4 [Epub ahead of print]
Gomez-Lechon MJ, Tolosa L, Conde I, Donato MT (2014) Competency of different cell models to predict human hepatotoxic drugs. Expert Opin Drug Metab Toxicol 10(11):1553–1568. doi:10.1517/17425255.2014.967680
Guillouzo A, Corlu A, Aninat C, Glaise D, Morel F, Guguen-Guillouzo C (2007) The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem Biol Interact 168(1):66–73. doi:10.1016/j.cbi.2006.12.003
Hay DC, Zhao D, Fletcher J et al (2008) Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells 26(4):894–902. doi:10.1634/stemcells.2007-0718
He L, Vasiliou K, Nebert DW (2009) Analysis and update of the human solute carrier (SLC) gene superfamily. Hum Genom 3(2):195–206. doi:10.1186/1479-7364-3-2-195
Hengstler JG, Brulport M, Schormann W et al (2005) Generation of human hepatocytes by stem cell technology: definition of the hepatocyte. Expert Opin Drug Metab Toxicol 1(1):61–74. doi:10.1517/17425255.1.1.61
Hewitt NJ, Lechon MJ, Houston JB et al (2007) Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev 39(1):159–234. doi:10.1080/03602530601093489
Kang M, Piliszek A, Artus J, Hadjantonakis AK (2013) FGF4 is required for lineage restriction and salt-and-pepper distribution of primitive endoderm factors but not their initial expression in the mouse. Development 140(2):267–279. doi:10.1242/dev.084996
Kola I, Landis J (2004) Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 3(8):711–715. doi:10.1038/nrd1470
Kubo A, Kim YH, Irion S et al (2010) The homeobox gene Hex regulates hepatocyte differentiation from embryonic stem cell-derived endoderm. Hepatology 51(2):633–641. doi:10.1002/hep.23293
La Rocca G, Anzalone R, Corrao S et al (2009) Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol 131(2):267–282. doi:10.1007/s00418-008-0519-3
Lee HJ, Jung J, Cho KJ, Lee CK, Hwang SG, Kim GJ (2012) Comparison of in vitro hepatogenic differentiation potential between various placenta-derived stem cells and other adult stem cells as an alternative source of functional hepatocytes. Differentiation 84(3):223–231. doi:10.1016/j.diff.2012.05.007
Leite SB, Teixeira AP, Miranda JP et al (2011) Merging bioreactor technology with 3D hepatocyte-fibroblast culturing approaches: improved in vitro models for toxicological applications. Toxicol In Vitro 25(4):825–832. doi:10.1016/j.tiv.2011.02.002
Li X, Yuan J, Li W et al (2014) Direct differentiation of homogeneous human adipose stem cells into functional hepatocytes by mimicking liver embryogenesis. J Cell Physiol 229(6):801–812. doi:10.1002/jcp.24501
Liu WH, Liu ZC, You N et al (2012) Several important in vitro improvements in the amplification, differentiation and tracing of fetal liver stem/progenitor cells. PLoS ONE 7(10):e47346. doi:10.1371/journal.pone.0047346
Martins JP, Santos JM, Almeida JM et al (2014) Towards an advanced therapy medicinal product based on mesenchymal stromal cells isolated from the umbilical cord tissue: quality and safety data. Stem Cell Res Ther 5:9. doi:10.1186/scrt398
Miranda JP, Leite SB, Muller-Vieira U, Rodrigues A, Carrondo MJ, Alves PM (2009) Towards an extended functional hepatocyte in vitro culture. Tissue Eng Part C Methods 15(2):157–167. doi:10.1089/ten.tec.2008.0352
Miranda J, Rodrigues A, Tostoes R et al (2010) Extending hepatocyte functionality for drug-testing applications using high-viscosity alginate-encapsulated three-dimensional cultures in bioreactors. Tissue Eng Part C Methods 16(6):1223–1232. doi:10.1089/ten.TEC.2009.0784
Miranda JP, Filipe E, Fernandes AS et al (2015) The human umbilical cord tissue-derived MSC population UCX® promotes early motogenic effects on keratinocytes and fibroblasts and G-CSF-mediated mobilization of BM-MSCs when transplanted in vivo. Cell Transplant 24(5):865–877. doi:10.3727/096368913X676231
Oliveros JC (2007–2015) Venny. An interactive tool for comparing lists with Venn's diagrams. http://bioinfogp.cnb.csic.es/tools/venny/index.html
Pal R, Mamidi MK, Das AK, Bhonde R (2012) Diverse effects of dimethyl sulfoxide (DMSO) on the differentiation potential of human embryonic stem cells. Arch Toxicol 86(4):651–661. doi:10.1007/s00204-011-0782-2
Rajan N, Habermehl J, Cote MF, Doillon CJ, Mantovani D (2006) Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat Protoc 1(6):2753–2758. doi:10.1038/nprot.2006.430
Rubbini S, Cocco L, Manzoli L et al (1997) Phosphoinositide signalling in nuclei of Friend cells: DMSO-induced differentiation reduces the association of phosphatidylinositol-transfer protein with the nucleus. Biochem Biophys Res Commun 230(2):302–305. doi:10.1006/bbrc.1996.5950
Rui L (2014) Energy metabolism in the liver. Compr Physiol 4(1):177–197. doi:10.1002/cphy.c130024
Santos JMS, Soares R, Martins JP, et al (2008) Optimised and defined method for isolation and preservation of precursor cells from human umbilical cord, INPI PAT20081000083882; PCT/IB2008/054067; WO2009044379. Medinfar, ECBio. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2009044379
Santos JM, Barcia RN, Simões SI et al (2013) The role of human umbilical cord tissue-derived mesenchymal stromal cells (UCX®) in the treatment of inflammatory arthritis. J Transl Med 11:18. doi:10.1186/1479-5876-11-18
Santos JM, Camões SP, Filipe E et al (2015) Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res Ther 6:90. doi:10.1186/s13287-015-0082-5
Schwartz RE, Fleming HE, Khetani SR, Bhatia SN (2014) Pluripotent stem cell-derived hepatocyte-like cells. Biotechnol Adv 32(2):504–513. doi:10.1016/j.biotechadv.2014.01.003
Seeliger C, Culmes M, Schyschka L et al (2013) Decrease of global methylation improves significantly hepatic differentiation of Ad-MSCs: possible future application for urea detoxification. Cell Transplant 22(1):119–131. doi:10.3727/096368912X638946
Sjogren AK, Liljevald M, Glinghammar B et al (2014) Critical differences in toxicity mechanisms in induced pluripotent stem cell-derived hepatocytes, hepatic cell lines and primary hepatocytes. Arch Toxicol 88(7):1427–1437. doi:10.1007/s00204-014-1265-z
Snykers S, Vanhaecke T, Papeleu P et al (2006) Sequential exposure to cytokines reflecting embryogenesis: the key for in vitro differentiation of adult bone marrow stem cells into functional hepatocyte-like cells. Toxicol Sci 94(2):330–341. doi:10.1093/toxsci/kfl058
Snykers S, Vanhaecke T, De Becker A et al (2007) Chromatin remodeling agent trichostatin A: a key-factor in the hepatic differentiation of human mesenchymal stem cells derived of adult bone marrow. BMC Dev Biol 7:24. doi:10.1186/1471-213X-7-24
Su T, Waxman DJ (2004) Impact of dimethyl sulfoxide on expression of nuclear receptors and drug-inducible cytochromes P450 in primary rat hepatocytes. Arch Biochem Biophys 424(2):226–234. doi:10.1016/j.abb.2004.02.008
Ulvestad M, Nordell P, Asplund A et al (2013) Drug metabolizing enzyme and transporter protein profiles of hepatocytes derived from human embryonic and induced pluripotent stem cells. Biochem Pharmacol 86(5):691–702. doi:10.1016/j.bcp.2013.06.029
Vyhlidal CA, Gaedigk R, Leeder JS (2006) Nuclear receptor expression in fetal and pediatric liver: correlation with CYP3A expression. Drug Metab Dispos 34(1):131–137. doi:10.1124/dmd.105.005967
Warnes GR, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw A, Lumley T, Maechler M, Magnusson A, Moeller S, Schwartz M (2009) gplots: Various R programming tools for plotting data. R Package Version 2(4)
Wilke RA, Lin DW, Roden DM et al (2007) Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat Rev Drug Discov 6(11):904–916. doi:10.1038/nrd2423
Wilkening S, Stahl F, Bader A (2003) Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug Metab Dispos 31(8):1035–1042. doi:10.1124/dmd.31.8.1035
Yen A, Varvayanis S (1995) DMSO, sodium butyrate, and TPA induce hypophosphorylation of RB with HL-60 cell differentiation. In Vitro Cell Dev Biol Anim 31(3):164–167. doi:10.1007/BF02639427
Yoon HH, Jung BY, Seo YK, Song KY, Park JK (2010) In vitro hepatic differentiation of umbilical cord-derived mesenchymal stem cell. Process Biochem 45(12):1857–1864. doi:10.1016/J.Procbio.2010.06.009
Yoshida Y, Shimomura T, Sakabe T et al (2007) A role of Wnt/beta-catenin signals in hepatic fate specification of human umbilical cord blood-derived mesenchymal stem cells. Am J Physiol Gastrointest Liver Physiol 293(5):G1089–G1098. doi:10.1152/ajpgi.00187.2007
Zhang W, Yatskievych TA, Baker RK, Antin PB (2004) Regulation of Hex gene expression and initial stages of avian hepatogenesis by Bmp and Fgf signaling. Dev Biol 268(2):312–326. doi:10.1016/j.ydbio.2004.01.019
Zhang YN, Lie PC, Wei X (2009) Differentiation of mesenchymal stromal cells derived from umbilical cord Wharton’s jelly into hepatocyte-like cells. Cytotherapy 11(5):548–558. doi:10.1080/14653240903051533
Zhao R, Watt AJ, Li J, Luebke-Wheeler J, Morrisey EE, Duncan SA (2005) GATA6 is essential for embryonic development of the liver but dispensable for early heart formation. Mol Cell Biol 25(7):2622–2631. doi:10.1128/MCB.25.7.2622-2631.2005
Zhou R, Li Z, He C et al (2014) Human umbilical cord mesenchymal stem cells and derived hepatocyte-like cells exhibit similar therapeutic effects on an acute liver failure mouse model. PLoS ONE 9(8):e104392. doi:10.1371/journal.pone.0104392
Acknowledgements
The authors thank Alexandra Medeiros for technical support on immunochemistry assays. This work was supported by Fundação para a Ciência e a Tecnologia (FCT, Portugal) through research grants (PTDC/SAU-TOX/110457/2009, PEst-OE/SAU/UI4013/2011, UID/DTP/04138/2013, SFRH/BD/87508/2012 to M.C., PD/BD/114280/2016 to S.P.C. and SFRH/BPD/96719/2013 and IF/00846/2015 to J.P.M.) and by Human Frontier Science Program (Young Investigator grant to J.L.R). We also acknowledge the support of the COST action BM1305 (A FACTT: Action to focus and accelerate cell-based tolerance-inducing therapies). The work herein presented was performed at iMed.ULisboa and Karolinska Institutet.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H.C. and P.C. are shareholders of ECBio; J.M.S. is employee of ECBio. The other authors declare that they have no conflict of interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of the Hospital Dr. José de Almeida (Cascais, Portugal), in scope of a research protocol between ECBio (Research & Development in Biotechnology, S.A.) and HPP Saúde (Parcerias Cascais, S.A.). Umbilical cord donations, with written informed consents, as well as umbilical cord procurement, were made according to Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurements, testing, processing, preservation, storage and distribution of human tissues and cells. All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cipriano, M., Correia, J.C., Camões, S.P. et al. The role of epigenetic modifiers in extended cultures of functional hepatocyte-like cells derived from human neonatal mesenchymal stem cells. Arch Toxicol 91, 2469–2489 (2017). https://doi.org/10.1007/s00204-016-1901-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1901-x