Abstract
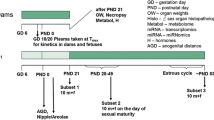
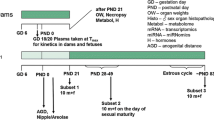
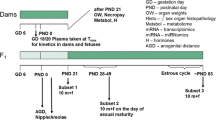
The current investigation examines whether the model anti-androgenic substance flutamide is capable of disrupting endocrine homeostasis at very low doses. The data generated clarify whether a non-monotonic dose–response relationship exists to enhance the current debate about the regulation of endocrine disruptors. Moreover, it is part of a series of investigations assessing the dose–response relationship of single and combined administration of anti-androgenic substances. A pre–postnatal in vivo study design was chosen, which was compliant with regulatory testing protocols. The test design was improved by additional endpoints addressing hormone levels, morphology, and histopathological examinations. Doses were chosen to represent a clear effect level (2.5 mg/kg bw/d), a low endocrine effect level (LOAEL, 0.25 mg/kg bw/d), a NOAEL for endocrine effects (0.025 mg/kg bw/d), a further dose at 0.0025 mg/kg bw/d flutamide, as well as an “ADI” (0.00025 mg/kg bw/d or 100-fold below the NOAEL) for the detection of a possible non-monotonic dose–response curve. Anti-androgenic changes were observable at LOAEL and the clear effect dose level but not at lower exposures. Nipple retention appeared to be the most sensitive measure of anti-androgenic effects, followed by age at sexual maturation, anogenital distance/anogenital index and male sex organ weights, as well as gross and histopathological findings. The results of all five doses indicate the absence of evidence for effects at very low dose levels. A non-monotonic dose–response relationship was not evident for the anti-androgenic drug flutamide.







Similar content being viewed by others
References
Adamsson NA, Brokken LJS, Paranko J, Toppari J (2008) In vivo and in vitro effects of flutamide and diethylstilbestrol on fetal testicular steroidogenesis in the rat. Reprod Toxicol 25:76–83
Borgert CJ, Stuchal LD, Mihaich EM, Becker RA, Bentley KS, Brausch JM, Coady K, Geter DR, Gordon E, Guiney PD, Hess F, Holmes CM, LeBaron MJ, Levine S, Marty S, Mukhi S, Neal BH, Ortego LS, Saltmiras DA, Snajdr S, Staveley J, Tobia A (2014) Relevance weighting of Tier 1 endocrine screening endpoints by rank order. Birth Defects Res B Dev Reprod Toxicol 101:90–113
Calabrese EJ, Blain R (2005) The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol 202:289–301
Carney EW, Zablotny CL, Marty MS, Crissman JW, Anderson P, Woolhiser M, Holsapple M (2004) The effects of feed restriction during in utero and postnatal development in rats. Toxicol Sci 82:237–249
Chernoff N, Gage MI, Stoker TE, Cooper RL, Gilbert ME, Rogers EH (2009) Reproductive effects of maternal and pre-weaning undernutrition in rat offspring: age at puberty, onset of female reproductive senescence and intergenerational pup growth and viability. Reprod Toxicol 28:489–494
Christiansen S, Boberg J, Axelstad M, Dalgaard M, Vinggaard AM, Metzdorff SB, Hass U (2010) Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reprod Toxicol 30:313–321
Delemarre-Van de Waal HA, van Coeverden SCCM, Engelbregt MJT (2002) Factors affecting onset of puberty. Horm Res 57:15–18
Diel P, Schmidt S, Vollmer G, Janning P, Upmeier A, Michna H, Bolt HM, Degen GH (2004) Comparative responses of three rat strains (DA/Han, Sprague–Dawley and Wistar) to treatment with environmental estrogens. Arch Toxicol 78:183–193
Dunger DB, Ahmed ML, Ong KK (2006) Early and late weight gain and the timing of puberty. Mol Cell Endocrinol 254–255:140–145
Fegert I, Billington R, Botham P, Carney E, FitzGerald RE, Hanley T, Lewis R, Marty MS, Schneider S, Sheets LP, Stahl B, van Ravenzwaay B (2012) Feasibility of the extended one-generation reproductive toxicity study (OECD 443). Reprod Toxicol 34:331–339
Foster PMD, McIntyre BS (2002) Endocrine active agents: implications of adverse and non-adverse changes. Toxicol Pathol 30:59–65
Goodman JE, Dodge DG, Bailey LA (2010) A framework for assessing causality and adverse effects in humans with a case study of sulfur dioxide. Regul Toxicol Pharmacol 58:308–322
Gray LE, Ostby J, Monosson E, Kelce WR (1999a) Environmental antiandrogens: low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol Ind Health 15:48–64
Gray LE, Wolf C, Lambright C, Mann P, Price M, Cooper RL, Ostby J (1999b) Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p, p′-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health 15:94–118
Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L (2001) Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update 7:248–264
Hellwig J, van Ravenzwaay B, Mayer M, Gembardt C (2000) Pre- and postnatal oral toxicity of vinclozolin in Wistar and Long-Evans rats. Regul Toxicol Pharmacol 32:42–50
Holsapple MR, Wallace KB (2008) Dose response considerations in risk assessment—an overview of recent ILSI activities. Toxicol Lett 180:85–92
Howdeshell KL, Rider CV, Wilson VS, Gray LE (2008) Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Res 108:168–176
ICH (2005) Study for effects on pre- and postnatal development, including maternal function. In: ICH Harmonized Tripartite Guideline for the Detection of toxicity to reproduction for medicinal products and toxicity to male fertility S5(R2) (International conference on harmonization of technical requirements for registration of pharmaceuticals for human use, ed), pp 8–15
Imperato-McGinley J, Sanchez RS, Spencer JR, Yee B, Vaughan ED (1992) Comparison of the effects of the 5-alpha-reductase inhibitor finasteride and the antiandrogen flutamide on prostate and genital differentiation—dose-response studies. Endocrinology 131:1149–1156
Kalil B, Leite CM, Carvalho-Lima M, Anselmo-Franci JA (2013) Role of sex steroids in progesterone and corticosterone response to acute restraint stress in rats: sex differences. Stress 16:452–460
Klimisch HJ, Andreae M, Tillmann U (1997) A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Regul Toxicol Pharmacol 25:1–5
Kortenkamp A, Martin O, Faust M, Evans R, McKinlay R, Orton F, Rosivatz E (2011) State of the art assessment of endocrine disrupters final report. 070307/2009/550687/SER/D3
Laier P, Metzdorff SB, Borch J, Hagen ML, Hass U, Christiansen S, Axelstad M, Kledal T, Dalgaard M, McKinnell C, Brokken LJS, Vinggaard AM (2006) Mechanisms of action underlying the antiandrogenic effects of the fungicide prochloraz. Toxicol Appl Pharmacol 213:160–171
Lau C, Klinefelter G, Cameron AM (1996) Reproductive development and functions in the rat after repeated maternal deprivation stress. Fundam Appl Toxicol 30:298–301
Laws SC, Stoker TE, Ferrell JM, Hotchkiss MG, Cooper RL (2007) Effects of altered food intake during pubertal development in male and female Wistar rats. Toxicol Sci 100:194–202
Marty MS, Johnson KA, Carney EW (2003) Effect of feed restriction on Hershberger and pubertal male assay endpoints. Birth Defects Res B Dev Reprod Toxicol 68:363–374
McIntyre BS, Barlow NJ, Foster PMD (2001) Androgen-mediated development in male rat offspring exposed to flutamide in utero: permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicol Sci 62:236–249
Melching-Kollmuss S, Fussell KC, Buesen R, Dammann M, Schneider S, Tennekes H, van Ravenzwaay B (2014) Anti-androgenicity can only be evaluated using a weight of evidence approach. Regul Toxicol Pharmacol 68:175–192
Mikkila TFM, Toppari J, Paranko J (2006) Effects of neonatal exposure to 4-tert-octylphenol, diethylstilbestrol, and flutamide on steroidogenesis in infantile rat testis. Toxicol Sci 91:456–466
O’Connor JC, Cook JC, Slone TW, Makovec GT, Frame SR, Davis LG (1998) An ongoing validation of a Tier I screening battery for detecting endocrine-active compounds (EACs). Toxicol Sci 46:45–60
OECD (2002) Appraisal of test methods for sex hormone disruption chemicals. ENV/JM/MONO(2002)8
OECD Guidance document (GD) on standardized test guidelines for evaluating chemicals for endocrine disruption: case studies using example chemicals. ENV/JM/MONO(2012)34
OECD guidance document for histologic evaluation of endocrine and reproductive tests in rodents. ENV/JM/MONO(2009)11. 28-7-2009
OECD guideline for the testing of chemicals 414: prenatal developmental toxicity study. OECD 414. 22-1-2001
OECD guideline for the testing of chemicals 443: extended one-generation reproductive toxicity study. OECD 443. 28-7-2011
Rhomberg LR, Goodman JE (2012) Low-dose effects and nonmonotonic dose-responses of endocrine disrupting chemicals: has the case been made? Regul Toxicol Pharmacol 64:130–133
Salewski E (1964) Färbemethode zum makroskopischen Nachweis von Implantationsstellen am Uterus der Ratte. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol 247:367–368
Schneider S, Kaufmann W, Strauss V, van Ravenzwaay B (2011) Vinclozolin: a feasibility and sensitivity study of the ILSI-HESI F1-extended one-generation rat reproduction protocol. Regul Toxicol Pharmacol 59:91–100
Toppari J, Skakkebaek NE (1998) Sexual differentiation and environmental endocrine disrupters. Baillieres Clin Endocrinol Metab 12:143–156
US Congress and US Environmental Protection Agency (1972) US Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA): Good Laboratory Practice Standards. 40 Code of Federal Regulations Part 160
US Congress and US Environmental Protection Agency (1976) US Toxic Substances Control Act (TSCA): Good Laboratory Practice Standards. 40 Code of Federal Regulations Part 792
US Environmental Protection Agency (1998) US EPA Health Effects Test Guideline: OPPTS 870.3700 Prenatal Developmental Toxicity Study. EPA-HQ-OPPT-2009-0156-0017
van Ravenzwaay B, Kolle SN, Ramirez T, Kamp HG (2013) Vinclozolin: a case study on the identification of endocrine active substances in the past and a future perspective. Toxicol Lett 223:271–279
van Weissenbruch MM, Engelbregt MJ, Veening MA, Delemarre-Van de Waal HA (2005) Fetal nutrition and timing of puberty. Endocr Dev 8:15–33
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP (2012) Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33:378–455
Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, Myers JP, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT (2013) Regulatory decisions on endocrine disrupting chemicals should be based on the principles of endocrinology. Reprod Toxicol 38:1–15
Viguiermartinez MC, Dereviers MTH, Barenton B, Perreaun C (1983) Effect of a non-steroidal anti-androgen, flutamide, on the hypothalamo-pituitary axis, genital-tract and testis in growing male-rats—endocrinological and histological data. Acta Endocrinol 102:299–306
Warren MP (1983) Effects of undernutrition on reproductive function in the human. Endocr Rev 4:363–377
Welsh M, MacLeod DJ, Walker M, Smith LB, Sharpe RM (2010) Critical androgen-sensitive periods of rat penis and clitoris development. Int J Androl 33:E144–E152
WHO/IPCS (1998) International programme on chemical safety, Report of IPCS/OECD scoping meeting on endocrine disrupters (EDCs), Washington
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fussell, K.C., Schneider, S., Buesen, R. et al. Investigations of putative reproductive toxicity of low-dose exposures to flutamide in Wistar rats. Arch Toxicol 89, 2385–2402 (2015). https://doi.org/10.1007/s00204-015-1622-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1622-6