Abstract
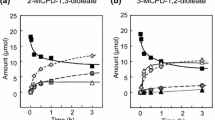
3-Chloro-1,2-propanediol (3-MCPD) fatty acid esters are formed upon thermal processing of fat-containing foods in the presence of chloride ions. Upon hydrolytic cleavage, these substances could release free 3-MCPD. This compound is toxicologically well characterised and displayed cancerogenic potential in rodent models. Recently, serious contaminations of different food products with 3-MCPD fatty acid esters have been reported. In regard to a risk assessment, the key question is to which degree these 3-MCPD fatty acid esters are hydrolysed in the human gut. Therefore, the aim of the present project was to examine the hydrolysis of 3-MCPD fatty acid esters and the resulting release of free 3-MCPD by using differentiated Caco-2 cells, a cellular in vitro model for the human intestinal barrier. Here, we show that 3-MCPD fatty acid esters at a concentration of 100 μM were neither absorbed by the cells nor the esters were transported via a Caco-2 monolayer. 3-MCPD-1-monoesters were hydrolysed in the presence of Caco-2 cells. In contrast, a 3-MCPD-1,2-diester used in this study was obviously absorbed and metabolised by the cells. Free 3-MCPD was not absorbed by the cells, but the substance migrated through a Caco-2 monolayer by paracellular diffusion. From these in vitro studies, we conclude that 3-MCPD-1-monoesters are likely to be hydrolysed in the human intestine, thereby increasing the burden with free 3-MCPD. In contrast, intestinal cells seem to have the capacity to metabolise 3-MCPD diesters, thereby detoxifying the 3-MCPD moiety.



Similar content being viewed by others
References
Aoki J, Inoue A, Makide K, Saiki N, Arai H (2007) Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie 89:197–204
Artursson P, Palm K, Luthman K (2001) Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev 46:27–43
Asai M, Higuchi S, Kubota M, Iguchi K, Usui S, Hirano K (2006) Regulators for blood glucose level affect gene expression of aquaporin 3. Biol Pharm Bull 29:991–996
Ballard ST, Hunter JH, Taylor AE (1995) Regulation of tight-junction permeability during nutrient absorption across the intestinal epithelium. Annu Rev Nutr 15:35–55
Buesen R, Mock M, Seidel A, Jacob J, Lampen A (2002) Interaction between metabolism and transport of benzo[a]pyrene and its metabolites in enterocytes. Toxicol Appl Pharmacol 183:168–178
Chateau D, Pauquai T, Delers F, Rousset M, Chambaz J, Demignot S (2005) Lipid micelles stimulate the secretion of triglyceride-enriched apolipoprotein B48-containing lipoproteins by Caco-2 cells. J Cell Physiol 202:767–776
Cho WS, Han BS, Nam KT, Park K, Choi M, Kim SH, Jeong J, Jang DD (2008a) Carcinogenicity study of 3-monochloropropane-1, 2-diol in Sprague-Dawley rats. Food Chem Toxicol 46:3172–3177
Cho WS, Han BS, Lee H, Kim C, Nam KT, Park K, Choi M, Kim SJ, Kim SH, Jeong J, Jang DD (2008b) Subchronic toxicity study of 3-monochloropropane-1,2-diol administered by drinking water to B6C3F1 mice. Food Chem Toxicol 46:1666–1673
Cogburn JN, Donovan MG, Schasteen CS (1991) A model of human small intestinal absorptive cells. 1. Transport barrier. Pharm Res 8:210–216
Collier PD, Cromie DDO, Davies AP (1991) Mechanism of formation of chloropropanols present in protein hydrolysates. J AOCS 68:785–790
Deutsche Gesellschaft für Fettwissenschaft: DGF Standard Method C III 18 (2009) Ester-bound 3-chloropropane-1,2-diol (3-MCPD esters) and glycidol (glycidyl esters). Determination in fats and oils by GC-MS. Deutsche Einheitsmethoden zur Untersuchung von Fetten, Fettprodukten, Tensiden und verwandten Stoffen, Wissenschaftliche Verlagsgesellschaft, Stuttgart (Germany)
Divinova V, Svejkovska B, Dolezal M, Velísek J (2004) Determination of free and bound 3-Chloropropane-1, 2-diol by gas chromatography with mass spectrometric detection using deuterated 3-chloropropane-1,2-diol as internal standard. Czech J Food Sci 22:182–189
Ebert B, Seidel A, Lampen A (2005) Identification of BCRP as transporter of benzo[a]pyrene conjugates metabolically formed in Caco-2 cells and its induction by Ah-receptor agonists. Carcinogenesis 26:1754–1763
El Ramy R, Ould Elhkim M, Lezmi S, Poul JM (2007) Evaluation of the genotoxic potential of 3-monochloropropane-1,2-diol (3-MCPD) and its metabolites, glycidol and beta-chlorolactic acid, using the single cell gel/comet assay. Food Chem Toxicol 45:41–48
European Commission (2001) Commission Regulation No 466/2001. Setting maximum levels for certain contaminants in foodstuffs. Off J Eur Communities 2001(L364):5–24
Federal Institute for Risk Assessment (2007) BfR opinion 047/2007. Infant formula and follow-up formula may contain harmful 3-MCPD fatty acid esters. http://www.bfr.bund.de/cm/245/infant_formula_and_follow_up_formula_may_contain_harmful_3_mcpd_fatty_acid_esters.pdf
Hamlet CG, Sadd PA (2004) Chloropropanols and their esters in baked cereal products. Czech J Food Sci 22:259–262
Hamlet CG, Sadd PA, Crews C, Velísek J, Baxter DE (2002) Occurrence of 3-chloro-propane-1,2-diol (3-MCPD) and related compounds in foods: a review. Food Addit Contam 19:619–631
Jones AR, Gadiel P, Stevenson D (1981) The fate of oxalic acid in the Wistar rat. Xenobiotica 11:385–390
Levin MS, Talkad VD, Gordon JI, Stenson WF (1992) Trafficking of exogenous fatty acids within Caco-2 cells. J Lipid Res 33:9–19
Liao W, Chan L (2000) Apolipoprotein B, a paradigm for proteins regulated by intracellular degradation, does not undergo intracellular degradation in CaCo2 cells. J Biol Chem 275:3950–3956
Pinto M, Robine-Leon S, Appay MD, Kedinger N, Triadou N, Dussaulx E, Lacroix P, Simon-Assman K, Haffen K, Fogh J, Zwiebaum A (1983) Enterocyte like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell 47:323–330
Robjohns S, Marshall R, Fellows M, Kowalczyk G (2003) In vivo genotoxicity studies with 3-monochloropropan-1,2-diol. Mutagenesis 18:401–404
Seefelder W, Varga N, Studer A, Williamson G, Scanlan FP, Stadler RH (2008) Esters of 3-chloro-1,2-propanediol (3-MCPD) in vegetable oils: significance in the formation of 3-MCPD. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25:391–400
Spalinger JH, Seidman EG, Ménard D, Levy E (1998) Endogenous lipase activity in Caco-2 cells. Biochim Biophys Acta 1393:119–127
Sveikovská B, Novotny O, Divinova V, Reblova Z, Dolezal M, Velísek J (2004) Esters of 3-chloropropane-1, 2-diol in foodstuffs. Czech J Food Sci 22:190–196
Trotter PJ, Storch J (1993) Fatty acid esterification during differentiation of the human intestinal cell line Caco-2. J Biol Chem 268:10017–10023
Velísek J, Davídek J, Hajslová J, Kubelka V, Janícek G, Mánková B (1978) Chlorohydrins in protein hydrolysates. Z Lebensm Unters Forsch 167:241–244
Velísek J, Davídek J, Kubelka V, Janícek G, Svobodová Z, Simicová Z (1980) New chlorine-containing organic compounds in protein hydrolysates. J Agric Food Chem 28:1142–1144
Zelinková Z, Svejkovská B, Velísek J, Dolezal M (2006) Fatty acid esters of 3-chloropropane-1,2-diol in edible oils. Food Addit Contam 23:1290–1298
Acknowledgments
We thank Linda Brandenburger, Albrecht Maier and Dieter Köhl for technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buhrke, T., Weißhaar, R. & Lampen, A. Absorption and metabolism of the food contaminant 3-chloro-1,2-propanediol (3-MCPD) and its fatty acid esters by human intestinal Caco-2 cells. Arch Toxicol 85, 1201–1208 (2011). https://doi.org/10.1007/s00204-011-0657-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-011-0657-6