Abstract
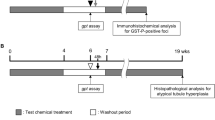
Fenofibrate (FF), a peroxisome proliferator-activated receptor-alpha agonist, has been used as one of the hypolipidemic drugs in man and induces oxidative stress and promotes hepatocarcinogenesis in the liver of rodents. This chemical belongs to a class of non-genotoxic carcinogens, but DNA damage secondary to oxidative stress resulting from reactive oxygen species (ROS) generation is suspected in rodents given this chemical. To examine whether FF has genotoxic potential, partially hepatectomized F344 male rats were treated orally with 0, 1,000 or 2,000 mg/kg of FF for 2 weeks, followed by diet containing 0.15% 2 acetyl aminofluorene (2 AAF) for enhancement the tumor-promoting effect for 10 days and a single oral dose of carbon tetrachloride (CCl4) as the first experiment (liver initiation assay). As the second experiment, the in vivo liver comet assay was performed in hepatectomized rats, and the expression of some DNA repair genes was examined. In the liver initiation assay, the number and area of glutathione S-transferase placental form (GST-P)-positive single cells and foci did not increase in the FF treated groups. In the comet assay, positive results were obtained after 3 h of the last treatment of FF, and the expression of some DNA repair genes such as Apex1, Ogg1 and Mlh1 were upregulated in rats given the high dose of FF at 3 h after the treatment but not in 24 h after the treatment. The results of the present study suggest that FF causes some DNA damage in livers of rats, but is not a strong genotoxic substance leading to a DNA mutation since such DNA damage was repaired by the increased activity of some DNA repair genes.





Similar content being viewed by others
References
Eacho PI, Lanier TC, Brodhecker CA (1991) Hepatocellular DNA synthesis in rats given peroxisome proliferating reagent: comparison of WY-14, 643 to clofibric acid, nafenopin and Ly171883 in rats. Carcinogenesis 12:1557–1561
Fairbairn DW, Oliv PL, O’ Neill KL (1995) The comet assays: a comprehensive review. Mutat Res 339:37–59
Guengerich FP, Liebler DC (1985) Enzymatic activation of chemicals to toxic metabolites. Crit Rev Toxicol 14:259–307
Hasegawa R, Yoshida T, Mizoguchi Y, Futakuchi M, Kim DJ, Cui L, Ito N (1994) Phenotypic alteration of hepatocellular foci in rats treated with clofibrate and phenobarbital. Cancer Lett 83:89–95
Hess R, Staubli W, Riess W (1965) Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy-isobutyrate in the rat. Nature 208:856–858
Hirata A, Tsukamoto T, Sakai H, Takasu S, Ban H, Imai T, Totsuka Y, Nishigaki R, Wakabayashi K, Yanai T, Masegi T, Tatematsu M (2008) Carcinogenic risk of heterocyclic amines in combination- assessment with a liver initiation model. Food Chem Toxicol 46:2003–2009
Jin M, Dewa Y, Kawai M, Nishimura J, Saegusa Y, Matsumoto S, Harada T, Shibutani M, Mitsumori K (2009) Induction of liver preneoplastic foci in F344 rats subjected to 28-day oral administration of diheptyl phthalate and its in vivo genotoxic potential. Toxicology 264:16–25
Kasai H (1997) Analysis of a form of oxidative DNA damage, 8-hydrox-2-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res 387:147–163
Klaunig JE, Kamendulis LM (2004) The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 44:239–267
Lefebvre P, Chinetti G, Fruchart JC, Staels B (2006) Sorting out the role of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest 116:571–580
Marsman DS, Cattley RC, Conway JG, Popp JA (1988) Relationship of hepatic peroxisome proliferation and replicative DNA synthesis to the hepatocarcinogenicity of the peroxisome proliferators di(2-ethylhexyl) phthalate and {4-chloro-6-(2, 3-xylidino)-2-pyrimidinylthio}acetic acid (Wy-14, 643) in rats. Cancer Res 48:6739–6744
Moto M, Sasaki Y, Okamura M, Fujita M, Kashida Y, Machida N, Mitsumori K (2003) Absence of in vivo genotoxicity and liver initiation activity of dicyclanil. J Toxicol Sci 28:173–179
Moto M, Umemura T, Okamura M, Muguruma M, Ito T, Jin M, Kashida Y, Mitsumori K (2006) Possible involvement of oxidative stress in dicyclanil-induced hepatocarcinogenesis in mice. Arch Toxicol 80:694–702
Muguruma M, Unami A, Kanki M, Kuroiwa Y, Nishimura J, Dewa Y, Umemura T, Oishi Y, Mitsumori K (2007) Possible involvement of oxidative stress in piperonyl butoxide induced hepatocarcinogenesis in rats. Toxicology 236:61–75
Nishikawa T, Wanibuchi H, Ogawa M, Kinoshita A, Morimura K, Hiroi T, Funae Y, Kishida H, Nakae D, Fukushima S (2002) Promoting effects of monomethylarsonic acid, dimethylarsinic acid and trimethylarsine oxide on induction of rat liver preneoplastic glutathione S-transferase placental form positive foci: a possible reactive oxygen species mechanism. Int J Cancer 100:136–139
Nishimura J, Dewa Y, Okamura T, Mugumura M, Jin M, Saegusa Y, Umemura T, Mitsumori K (2008) Possible involvement of oxidative stress in fenofibrate-induced hepatocarcinogenesis in rats. Arch Toxicol 82:641–654
Peters JM, Cheung C, Gonzalez FJ (2005) Peroxisome proliferator-activated receptor- alpha and liver cancer: where do we stand? J Mol Med 83:774–785
Prochaska HJ, Talalay P (1988) Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res 48:4776–4782
Puntarulo S, Cederbaum AI (1998) Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic Biol Med 24:1324–1330
Rao MS, Reddy JK (1987) Peroxisome proliferation and hepatocarcinogenesis. Carcinogenesis 8:631–636
Rao MS, Usuda N, Subbarao V, Reddy JK (1987) Absence of gamma-glutamyl transpeptidase activity in neoplastic lesions induced in the liver of male F-344 rats by di-(2 -ethylhexyl)phthalate, a peroxisome proliferator. Carcinogenesis 9:1347–1350
Rao MS, Nemali MR, Usuda N, Scarpelli DG, Makino T, Pitot HC, Reddy JK (1988) Lack of expression of glutathione-S-transferase P, gamma-glutamyl transpeptidase, and alpha-fetoprotein messenger RNAs in liver tumors induced by peroxisome proliferators. Cancer Res 48:4919–4925
Reddy JK, Rao MS (1989) Oxidative DNA damage caused by persistent peroxisome proliferation: it is role in hepatocarcinogenesis. Mutat Res 214:63–68
Reddy JK, Azarnoff DL, Hignite CE (1980) Hypolipidaemic hepatic peroxisome proliferators from a novel class of chemical carcinogens. Nature 283:397–398
Sakai H, Tsukamoto T, Yamamoto M, Yanai T, Masegi T, Inada K, Nakanishi H, Tatematsu M (2000) Summation of initiation activities of low doses of the non-hepatocarcinogen 1, 2-dimethylhydrazine in the liver after carbon tetrachloride administration. Cancer Lett 148:59–63
Sakai H, Tsukamoto T, Yamamoto M, Kobayashi K, Yuasa H, Imai T, Yanai T, Masegi T, Tatematsu M (2002) Distinction of carcinogens from mutagens by induction of liver cell foci in a model for detection of initiation activity. Cancer Lett 188:33–38
Sano M, Hagiwara A, Tamano S, Hasegawa R, Imaida K, Ito N, Shirai T (1999) Dose-dependent induction of carcinomas and glutathione S-transferase placental form negative eosinophilic foci in the rat liver by di(2-ethylhexyl)phathlate after diethylnitrosamine initiation. J Toxicol Sci 24:177–186
Sasaki YF, Nishidate E, Izumiyama F, Matsusaka N, Tsuda S (1997) Detection of rodent liver carcinogen genotoxicity by the alkaline single-cell gel electrophoresis (comet)assay in multiple mouse organs (liver, lung, spleen, kidney and bone marrow). Mutat Res 391:201–214
Seo KW, Kim KB, Kim YJ, Choi JY, Lee KT, Choi KS (2004) Comparison of oxidative stress and changes of xenobiotic metabolizing enzymes induced by phthalates in rats. Food Chem Toxicol 42:107–114
Shane BS, Smith-Dunn DL, Ganesh L, Cunningham ML (1999) Mutagenicity of Wyeth 14, 643 in vivo in the liver of Big Blue transgenic mice. Proc Am Assoc Cancer Res 39:241
Shane BS, Smith-Dunn DL, de Boer JG, Glickman BW, Cunningham ML (2000) Subchronic administration of phenobarbital alters the mutation spectrum of lacl in the livers of Big Blue transgenic mice. Mutat Res 448:69–80
Shoda T, Mitsumori K, Onodera H, Toyoda K, Uneyama C, Takada K, Hirose M (2000) Liver tumor-promoting effect of beta-naphthoflavone, a strong CYP 1A1/2 inducer, and the relationship between CYP 1A1/2 induction and Cx32 decrease in its hepatocarcinogenesis in the rat. Toxicol Pathol 28:540–547
Singh VK, Ganesh L, Cunningham ML, Shane BS (2001) Comparison of the mutant frequencies and mutation spectra of three non-genotoxic carcinogens, oxazepam, phenobarbital, and Wyeth 14, 643, at the lambdacll locus in Big Blue transgenic mice. Biochem Pharmacol 62:685–692
Staels B, Dallongeville J, Auwex J, Schoonjans K, Leitersdorf E, Frchart JC (1998) Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 98:2088–2093
Suzuki T, Jin M, Dewa Y, Ichimura R, Shimada Y, Mizukami S, Shibutani M, Mitsumori K (2010) Evaluation of in vivo liver genotoxic potential of Wy-14, 643 and piperonyl butoxide in rats subjected to two-week repeated oral administration. Arch Toxicol 84:493–500
Tsuda H, Lee G, Farber E (1980) Induction of resistant hepatocytes as a new principle for a possible short-term in vivo test for carcinogens. Cancer Res 40:1157–1164
US Food and Drug Administration (2008) http://www.fda.gov/medwatch/; search word is triglide tablets
Valavanidis A, Vlachogianni T, Fiotakis C (2009) 8-Hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 27:120–139
Woods CG, Burns AM, Bradford BU, Ross PK, Kosyk O, Swenberg JA, Cunninqham ML, Rusyn I (2007) WY-14, 643 induced cell proliferation and oxidative stress in mouse liver are independent of NADPH oxidase. Toxicol Sci 98:366–374
Yeldandi AV, Rao MS, Reedy JK (2000) Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutat Res 448:159–177
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tawfeeq, M.M., Suzuki, T., Shimamoto, K. et al. Evaluation of in vivo genotoxic potential of fenofibrate in rats subjected to two-week repeated oral administration. Arch Toxicol 85, 1003–1011 (2011). https://doi.org/10.1007/s00204-010-0628-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-010-0628-3