Abstract
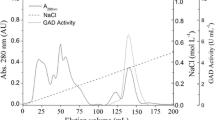
The enzyme 3-methylaspartase (3-methylaspartate ammonia-lyase, EC 4.3.1.2) was found in the cells of enteric bacteria, especially in the genera Citrobacter and Morganella, that were grown under anoxic and oxygen-limited conditions. The enzymes were purified to homogeneity from the cell-free extracts of 18 active strains and had similar enzymological properties such as action on columns, specific activity, molecular weight, subunit structure, and N-terminal amino acid sequence similarity. The production of the enzyme was dependent on the limitation of oxygen during growth and was arrested by aeration. The addition of external electron acceptors such as dimethylsulfoxide could support cell growth and production of the enzyme. Activities of glutamate mutase (EC 5.4.99.1) and (S)-citramalate hydrolyase (EC 4.2.1.34), key enzymes of the mesaconate pathway of (S)-glutamate fermentation in the genus Clostridium, were detected in the cells of the active strains grown under oxygen-limited conditions. Based on the results, the mesaconate pathway is proposed to explain the (S)-glutamate fermentation process observed in Enterobacteriaceae, and 3-methylaspartase could be a marker enzyme for this pathway.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 28 May 1997 / Accepted: 16 July 1997
Rights and permissions
About this article
Cite this article
Kato, Y., Asano, Y. 3-Methylaspartate ammonia-lyase as a marker enzyme of the mesaconate pathway for (S)-glutamate fermentation in Enterobacteriaceae. Arch Microbiol 168, 457–463 (1997). https://doi.org/10.1007/s002030050522
Issue Date:
DOI: https://doi.org/10.1007/s002030050522